Archived Content
The National Institute of Mental Health archives materials that are over 4 years old and no longer being updated. The content on this page is provided for historical reference purposes only and may not reflect current knowledge or information.
Circuit for Experience-Informed Decision-Making ID’d in Rats
Memory and executive hubs work in lockstep during awake mental replay
• Press Release
How is the brain able to use past experiences to guide decision-making? A few years ago, researchers supported by the National Institutes of Health discovered in rats that awake mental replay of past experiences is critical for learning and making informed choices. Now, the team has discovered key secrets of the underlying brain circuitry – including a unique system that encodes location during inactive periods.
“Advances such as these in understanding cellular and circuit-level processes underlying such basic functions as executive function, social cognition, and memory fit into NIMH’s mission of discovering the roots of complex behaviors,” said NIMH acting director Bruce Cuthbert, Ph.D.
While a rat is moving through a maze — or just mentally replaying the experience — an area in the brain’s memory hub, or hippocampus, specialized for locations, called CA1, communicates with a decision-making area in the executive hub or prefrontal cortex (PFC). A distinct subset of PFC neurons excited during mental replay of the experience are activated during movement, while another distinct subset, less engaged during movement in the maze – and therefore potentially distracting – are inhibited during replay.
“Such strongly coordinated activity within this CA1-PFC circuit during awake replay is likely to optimize the brain’s ability to consolidate memories and use them to decide on future action” explained Shantanu Jadhav, Ph.D., now an assistant professor at Brandeis University, Waltham, MA., the study’s co-first author. His contributions to this line of research were made possible, in part, by a Pathway to Independence award from the Office of Research Training and Career Development of the NIH’s National Institute of Mental Health (NIMH).
Jadhav and his fellow scientist Gideon Rothschild, Ph.D., led the study under the supervision of their post-doctoral preceptor, NIMH grantee Loren Frank, Ph.D. , of the University of California, San Francisco (UCSF). They report on their findings March 10, 2016 in the journal Neuron.
Prior to the study, researchers knew that the hippocampus and prefrontal cortex play a critical role in memory-guided behavior, but exactly what neural activity patterns underlie these abilities remained a mystery.
Previous studies had shown that neurons called place cells in the hippocampus become associated with particular places when rats explore mazes. During breaks when animals are inactive, they replay these place experiences in their minds. The place cells that activated while exploring the maze fire again in the same sequence, but on a much faster timescale. This is reflected in telltale split-second bursts of electrical activity called sharp-wave ripples (SWRs), in the hippocampus.
“We had previously shown that this SWR activity in the hippocampus is necessary for learning, but we didn’t know if or how it might engage other parts of the brain,” explained Jadhav. “We suspected that in order to support memory retrieval, hippocampal and PFC activity during SWRs has to be coordinated.”
When they examined activity in groups of neurons in the two regions simultaneously as rats were learning spatial tasks, Jadhav and Rothschild’s team saw coordinated reactivation during SWRs spanning both the hippocampus and the PFC. In the PFC, they were surprised to see that this reactivation involved both excitation as well as inhibition of functionally distinct populations of neurons. Within a particular SWR, prefrontal neurons that showed spatial representations similar to the concurrently reactivated hippocampal neurons were excited, whereas prefrontal neurons with unrelated representations were inhibited. Any potentially distracting activity inconsistent with the replayed information coming from the hippocampus would thus be suppressed, presumably optimizing awake memory function.
“Our results show that SWRs mark times of strong coordination between hippocampus and prefrontal cortex that reflects highly specific structured reactivation of representations related to ongoing experience,” said Jadhav.
In a parallel study, Frank’s UCSF team focused on a neighboring region of the hippocampus called CA2. There, they were surprised to discover a distinct population of neurons that not only fire most strongly outside of SWRs, but also signal an animal’s location when the animal is immobile, including during sleep. The firing pattern of these neurons was thus complementary to the firing pattern of the SWR-associated place cells found in CA1.
The study revealed that the brain employs distinct neural codes for formation of location-specific memories depending on whether the animal is moving or still. Notably, CA2 has also recently been linked to social memories; social experiences often take place during periods of inactivity, according to Frank.
Remarkably, the timescale of the firing changes from SWR- vs. non-SWR patterns were similar in the prefrontal cortex and in CA2, indicating that the brain rapidly switches between the two coding systems with split-second agility.
“Delusions and similar mental problems involve mistaking internally generated information for real things from the outside world; it may be that the rapid and precise switching between past and present that we see in normal brains is impaired in psychiatric disease,” said Frank.
Frank and a UCSF team led by graduate student Kenneth Kay – whose research training was also supported, in part, by an individual NIMH fellowship award – reported on their discoveries online March 2, 2016 in the journal Nature.
“Investing the next generation of research scientists, such as Jadhav and Kay, by supporting their mentored training in state-of-the-art research skills, is essential if the NIMH is to continue to accomplish our mission,” said Nancy Desmond, Ph.D., Associate Director for Research Training and Career Development in the NIMH Division of Neuroscience and Basic Behavioral Science.
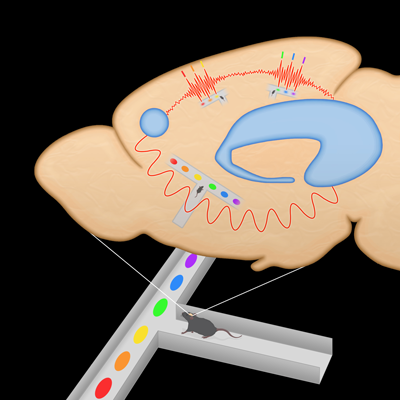
Brain circuitry underlying memory for location and experience-based decision-making has been discovered in rats.
Source: Shantanu Jadhav, Ph.D., Brandeis University
For more information, see:
'Brain GPS' Component Allows Brain to Track Location When at Rest (UCSF press release)
References
Coordinated Excitation and Inhibition of Prefrontal Ensembles During Awake Hippocampal Sharp-Wave Ripple Events. Jadhav SP, Rothschild G, Roumis DK, Frank LM. Neuron, March 10, 2016.
A hippocampal network for spatial coding during immobility and sleep. Kay K, Sosa M, Chung JE, Karlsson MP, Larkin MC, Frank LM. Nature. 2016 Mar 2. doi: 10.1038/nature17144. [Epub ahead of print] PMID: 26934224
Grants
MH090188, MH100284
About the National Institute of Mental Health (NIMH): The mission of the NIMH is to transform the understanding and treatment of mental illnesses through basic and clinical research, paving the way for prevention, recovery and cure. For more information, visit the NIMH website.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit the NIH website .
NIH…Turning Discovery Into Health®
