Archived Content
The National Institute of Mental Health archives materials that are over 4 years old and no longer being updated. The content on this page is provided for historical reference purposes only and may not reflect current knowledge or information.
Scientists Switch Neurons On and Off Using Light
Technique is valuable new tool in brain research
• Science Update
Researchers have invented a genetically-engineered way to turn the electrical impulses of brain cells on and off with pulses of blue and yellow light — in synch with the split-second pace of real time neuronal activity. The novel technique borrows genes from light-responsive algae and bacteria to unravel the intricate workings of brain circuits with extreme precision. Initial tests in intact mouse circuits and tiny living worms promise new hope for understanding — and eventually treating — psychiatric and neurological disorders.
In the future, brain disorders could potentially be managed with light-emitting implants, envisions NIMH grantee Dr. Karl Deisseroth, Stanford University, whose research team reports on their findings in the April 5, 2007 Nature.
“The wide variety of neuronal cell types involved in mental illness aren’t conveniently clumped together, but rather sparsely embedded in complex circuitry,” explained Deisseroth, an engineer as well as a psychiatrist who treats depressed patients.
Much brain circuit activity happens so quickly and at such a micro, neuron-specific level that it eludes detection even with the most advanced imaging technologies. Neuroscientists need more precise and reversible methods for activating and inactivating neurons in experimental models.
To address these issues, his team, which included German collaborators, created probes that selectively target particular types of neurons. They loaded a deactivated virus with genes from light-sensitive primitive organisms and fluorescent proteins or other markers. The loaded virus then carried the genes into specific neurons, where the genes began producing proteins necessary for turning the neurons on and off — just as they do in the primitive organisms.
To turn neurons on, they borrowed a gene (ChR2) from green algae that opens the flow of sodium into its single cell in response to blue light to control its movement towards light in order to conduct photosynthesis.
To turn neurons off, they adapted a gene (NpHR) from a primitive bacterium native to salt lakes in Egypt. In response to yellow light, it regulates salt balance by pumping chloride into its cell.
The internal balance of sodium and chloride determines when a neuron fires.
In one experiment in the study, cultured rat neurons that had been loaded with the ChR2 and NpHR genes fired in lockstep with pulses of blue light, but stopped firing in the presence of yellow light. The researchers have also demonstrated the same on/off control in living mouse circuitry and in tiny translucent worms, whose swimming behavior was stopped and started by targeting a specific class of motor neurons.
The researchers suggest that the new tools could be used with existing imaging approaches in living animals engaged in behaviors to pinpoint how neural activity patterns relate to brain-circuit functioning. Deisseroth said the tools may also find application in determining the therapeutic potential of experimental drugs.
He envisions the possibility, in the more distant future, of genetic targeting of neurons involved in disease processes. For example, light stimulation could potentially offer a more precise and less invasive alternative to electrical stimulation for treating disorders like Parkinson’s disease and depression.
“This accomplishment is a key step toward the important goal of mapping neural circuit dynamics on a millisecond timescale. This approach could provide new insights into the complex dynamics of brain function in a range of diseases,” said National Institutes of Health Director Elias A. Zerhouni, M.D. “The work is also a prime example of the highly innovative approaches to major challenges in biomedical research that we support through the NIH Director’s Pioneer Award program.”
Deisseroth is a recipient of a Pioneer Award . NIH provides a dozen or so such grants each year to “scientists of exceptional creativity” for “approaches that have the potential to produce an unusually high impact.” His work was also funded, in part, by the National Institute on Drug Abuse .
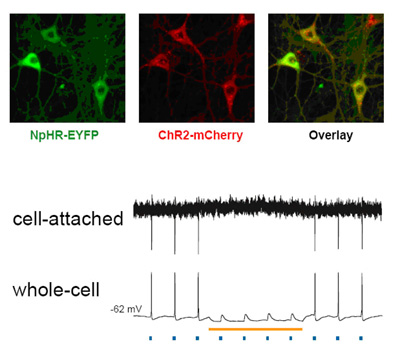
Activity of neurons infected with light-responsive genes was turned on by blue light and turned off by yellow light. Spikes of electrical activity in lockstep with pulses of blue light (blue dots) were suppressed in the presence of yellow light (yellow line). Expression of the activating gene ChR2 (red) and suppressing gene NpHR (green) and the two combined (overlay) is shown in cultured neurons from rat hippocampus.
Source: Karl Deisseroth, M.D., Ph.D, Stanford University

Pulses of blue and yellow light precisely turn neurons on-and-off using genetically-targeted probes that take advantage of light-sensitive genes borrowed from primitive life-forms. Artist’s rendering.
Source: Karl Deisseroth, M.D., Ph.D., Stanford University
References
*Zhang F, Wang LP, Brauner M, Liwald JF, Kay K, Watzke N, Wood P, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature, 4/5/07.
**Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity . Nat Neurosci. 2005 Sep;8(9):1263-8. Epub 2005 Aug 14. PMID: 16116447
