Oxytocin and its role in reproduction and behavior
by W. Scott Young
Summary
Oxytocin (OT) is a nonapeptide that was first identified in pituitary extracts through its effects on labor and milk secretion (1, 2, 3). Furthermore, the concept of neurosecretion arose from studies of OT and the other major neurohypophyseal hormone, the antidiuretic hormone vasopressin (VP) (4). The early determination of their amino acid sequences (5) permitted more detailed studies of their distributions and biological effects. The majority of both OT and VP were shown to be made in the magnocellular neurons of the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus (6, 7) and processed from their precursor forms along the axonal projection to the posterior pituitary (8). Subsequent cloning of the cDNAs encoding the bovine precursors for OT and VP (9, 10) opened the way for studies examining their levels of expression during various physiological and manipulated states. Further molecular biological studies revealed that the two genes are linked on the same chromosome and arose through gene duplication (11, 12).
The original observations linking OT with lactation and parturition have been been confirmed in numerous studies. For example, opioids inhibit parturition in rats by reducing neurohypophysial secretion of OT (13). In addition, other functions for OT have been discovered or suggested. These include roles in mating and maternal behaviors (14, 15, 16, 17, 18), natriuresis (19) and antidiuresis(20), and memory (21). Nevertheless, OT has not been shown to be necessary for successful procreation. Attempts to examine whether OT is absolutely required in the various steps from mating through pregnancy to rearing have generally used OT antagonists or antibodies or selective brain lesions(22, 23, 24, 25). The results of these studies have not been wholly consistent, have been confounded by the absence of other hormones, or reflect species differences. For example, OT antagonists prevent premature parturition in some patients, while other patients do not benefit from this form of therapy. Similarly, some rats escape inhibition by OT antagonists (26).
The importance of OT in males is much less well understood. OT expression and release are increased by hyperosmolar and stressful stimuli (27, 28, 29); reviewed in (30). In addition, OT in males induces natriuresis and inhibits sodium appetite (19, 31). Evidence also suggests that OT is involved in prolactin release (32); penile erection and yawning (33); maintaining sperm counts (34); and, especially, various behaviors including affiliative, aggressive, and sexual activities (reviewed in ref. (35). For example, i.c.v. OT shortens ejaculation latency and post-ejaculation interval of rats (36). Furthermore, OTR expression in the ventromedial nucleus of the hypothalamus of the male rat is up-regulated by estradiol and dihydrotestosterone (37). However, whether OT or its receptor are necessary for males to survive and procreate is unknown. Even less is known about any role for OT in development. OTRs are detected in fetal rats (38) prior to expression of OT (reviewed in ref. 30). Even after OT is expressed in the fetal rat brain, carboxy-terminal extended forms of OT are not completely processed to OT until after birth (39). It has been suggested that these extended forms of OT may act as vasopressin antagonists during development (39), but no evidence exist to support this hypothesis. So, the relationship between OT and its receptor is not always evident.
In order to determine the functions for which OT is required, gene targeting has been used to eliminate OT in mice (40, 41). These studies indicate that reproductive and maternal behaviors are essentially untouched, with both male and female homozygous knockouts being fertile and females capable of parturition. However, the pups die within 24 hours because they are unable to obtain milk from the dams. Oxytocin injections into the dams restores successful suckling by the pups. Further studies of these mice are currently underway to examine mice behaviors in more detail and to determine if the OT receptor is being activated by vasopressin to compensate in some instances for the lack of OT.
Figure 2
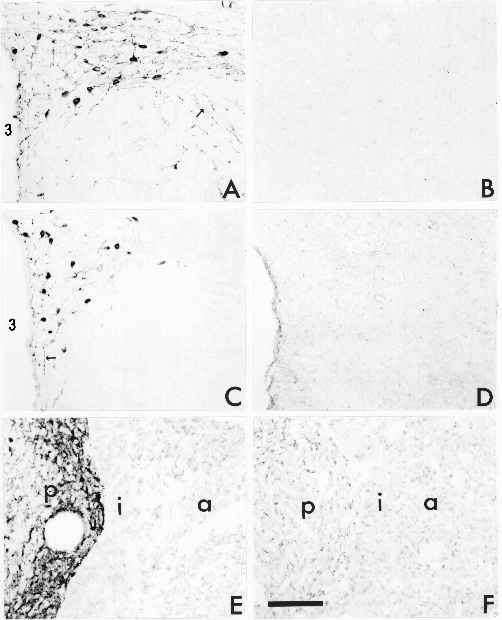
Legend
Immunohistochemical detection of OT neurophysin (A,B) or of processed OT (C-F) in the PVN (A-D) or pituitary (E-F) of wild-type (A, C, E) and homozygous mutant (B, D, F) mice. There are numerous heavily stained neurons and axon fibers (arrows) present in the wild-type mice stained with either antibody. No staining was observed with either the neurophysin or the OT antibody in the mutant mouse. OT immunoreactivity was abundant in the posterior pituitary (p) of the WT mouse, but absent from that of the HO mouse. 3 is the third ventricle, a is anterior pituitary, i is intermediate lobe. Bar equals 1mm. (Click within the figure to see enlarged, 24K, version)
References
- G. Oliver, E. A. Schäfer, J. Physiol. (Lond.) 18, 277-279 (1895).
- H. H. Dale, Biochem. J. 4, 427-447 (1909).
- I. Ott, J. C. Scott, Proc. Soc. Exp. Biol. Med. 8, 48-49 (1911).
- W. Bargmann, E. Scharrer, Am. Scientist 39, 255-259 (1951).
- V. Du Vigneaud, Harvey Lect. 50, 1-26 (1954).
- D. F. Swaab, C. W. Pool, F. Nijveldt, J. Neural Transmission 36, 195-215 (1975).
- F. Vandesande, K. Dierickx, Cell. Tiss. Res. 164, 153-162 (1975).
- M. J. Brownstein, J. T. Russell, H. Gainer, Science 207, 373-387 (1980).
- H. Land, G. Schutz, H. Schmale, D. Richter, Nature 295, 299-303 (1982).
- H. Land, et al., Nature 302, 342-344 (1983).
- E. Sausville, D. Carney, J. Battey, J. Biol. Chem. 260, 10236-10241 (1985).
- Y. Hara, J. Battey, H. Gainer, Mol. Brain Res. 8, 319-324 (1990).
- J. A. Russell, G. Leng, R. J. Bicknell, Exper. Physiol. 80, 307-340 (1995).
- C. A. Pederson, A. J. J. Prange, Proc. Natl. Acad. Sci. (USA) 76, 6661-6665 (1979).
- E. van Leengoed, E. Kerker, H. H. Swanson, J. Endocrinol. 112, 275-282 (1987).
- C. A. Pederson, J. D. Caldwell, G. F. Jirikowski, T. R. Insel, Eds., Oxytocin in Maternal, Sexual, and Social Behaviors, vol. 652 (New York Academy of Sciences, New York, 1992).
- T. Insel, Psychoneuroendocrinology 17, 3-35 (1992).
- D. M. Witt, Neurosci. Biobehav. Rev. 19, 315-324 (1995).
- J. G. Verbalis, M. P. Mangione, E. M. Stricker, Endocrinology 128, 1317-1322 (1991).
- C.-L. Chou, S. R. DiGiovanni, R. Mejia, S. Nielsen, M. A. Knepper, Am. J. Physiol. 269(1 Pt 2), F70-7 (1995).
- D. de Wied, J. Elands, G. Kovács, Proc. Natl. Acad. Sci. (USA) 88, 1494-1498 (1991).
- C. C. Gale, S. M. McCann, J. Endocrinol. 22, 107-117 (1961).
- P. Kumaresan, A. Kagan, S. M. Glick, Nature 230, 468-469 (1971).
- P. Melin, Baillieres Clin. Obstet. Gynaecol. 7, 577-600 (1993).
- T. M. Goodwin, et al., Am. J Obstet. Gynecol. 170, 474-8 (1994).
- I. A. Antonijevic, et al., J. Endocrinol. 145, 97-103 (1995).
- R. E. J. Dyball, J. Physiol. (Lond.) 214, 245-256 (1971).
- T. D. M. Williams, D. A. Carter, S. L. Lightman, Endocrinology 116, 738-740 (1985).
- R. Landgraf, I. Neumann, H. Schwarzberg, Brain Res. 457, 219-225 (1988).
- W. S. Young, III, J. Neuroendocrinol. 5, 527-540 (1992).
- J. G. Verbalis, R. E. Blackburn, B. R. Olson, E. M. Stricker, Regulat. Peptides 45, 149-154 (1993).
- M. D. Lumpkin, W. K. Samson, S. M. McCann, Endocrinology 112, 1711-1717 (1983).
- M. R. Melis, R. Stancampiano, A. Argiolas, Pharmacol. Biochem. Behav. 48, 203-207 (1994).
- A. Ågmo, R. Andersson, C. Johansson, Biol. Reprod. 18, 346-349 (1978).
- J. F. Leckman, et al., Psychoneuroendocrinology 19, 723-749 (1994).
- R. Arletti, A. Benellil, A. Bertolini, in Oxytocin in Maternal, Sexual, and Social Behaviors C. A. Pederson, J. D. Caldwell, G. F. Jirikowski, T. R. Insel, Eds. (New York Academy of Sciences, New York, 1992), vol. 652, pp. 180-193.
- A. E. Johnson, H. Coirini, T. R. Insel, B. S. McEwen, Endocrinology 128, 891-896 (1991).
- E. Tribollet, S. Charpak, A. Schmidt, M. Dubois-Dauphin, J. Dreifuss, J. Neurosci. 9, 1764-1773 (1989).
- M. Altstein, M. H. Whitnall, S. House, S. Key, H. Gainer, Peptides 9, 87-105 (1988).
- K. Nishimori, et al., Proc. Natl. Acad. Sci. (USA) 93, 11699-11704 (1996).
- W. S. Young, III, et al., J. Neuroendocrinol. 8, 847-853 (1996).
October of 1996
