Archived Content
The National Institute of Mental Health archives materials that are over 4 years old and no longer being updated. The content on this page is provided for historical reference purposes only and may not reflect current knowledge or information.
Perinatal Antidepressant Stunts Brain Development in Rats
Miswired brain circuitry traced to early exposure – NIH-funded study
• Press Release
Rats exposed to an antidepressant just before and after birth showed substantial brain abnormalities and behaviors, in a study funded by the National Institutes of Health.
After receiving citalopram, a serotonin-selective reuptake inhibitor (SSRI), during this critical period, long-distance connections between the two hemispheres of the brain showed stunted growth and degeneration. The animals also became excessively fearful when faced with new situations and failed to play normally with peers – behaviors reminiscent of novelty avoidance and social impairments seen in autism. The abnormalities were more pronounced in male than female rats, just as autism affects 3-4 times more boys than girls.
“Our findings underscore the importance of balanced serotonin levels – not too high or low -- for proper brain maturation,” explained Rick Lin, Ph.D ., of the University of Mississippi Medical Center, Jackson, a Eureka Award grantee of the NIH’s National Institute of Mental Health.
Lin and colleagues report on their discovery online during the week of Oct. 24, 2011, in the Proceedings of the National Academy of Sciences.
Last July, a study reported an association between mothers taking antidepressants and increased autism risk in their children. It found that children of mothers who took SSRI’s during the year prior to giving birth ran twice the normal risk of developing autism – with treatment during the first trimester of pregnancy showing the strongest effect. A study published last month linked the duration of a pregnant mother’s exposure to SSRIs to modest lags in coordination of movement – but within the normal range – in their newborns.
“While one must always be cautious extrapolating from medication effects in rats to medication effects in people, these new results suggest an opportunity to study the mechanisms by which antidepressants influence brain and behavioral development,” said NIMH Director Thomas R. Insel, M.D. “These studies will help to balance the mental health needs of pregnant mothers with possible increased risk to their offspring.”
Earlier studies had hinted that serotonin plays an important role in shaping the still-forming brain in the days just after a rat is born, which corresponds to the end of the third trimester of fetal development in humans. Experimental manipulations of the chemical messenger during this period interfered with formation of sensory-processing regions of the cortex, or outer mantle, and triggered aggressive and anxiety-related behaviors in rodents.
There is also recent evidence in humans that serotonin from the placenta helps shape development of the fetal brain early in pregnancy. Disrupted serotonin has been linked to mood and anxiety disorders. SSRIs, the mainstay medication treatment for these disorders, boost serotonin activity.
Lin and colleagues gave citalopram to male and female rat pups prenatally and postnatally and examined their brains and behavior as they grew up. Male, but not female, SSRI exposed rat pups abnormally froze when they heard an unfamiliar tone and balked at exploring their environment in the presence of unfamiliar objects or scents. These behaviors persisted into adulthood. The male pups especially also shunned normal juvenile play behavior – mimicking traits often seen in children with autism.
A key brain serotonin circuit, the raphe system, known to shape the developing brain during the critical period when the animals were exposed to the drug, showed dramatic reductions in density of neuronal fibers. Evidence of stunted development in the circuit coursed through much of the cortex and other regions important for thinking and emotion, such as the hippocampus.
The researchers also discovered miswiring in the structure responsible for communications between the brain’s left and right hemispheres, called the corpus collosum. Extensions of neurons, called axons, through which such long-distance communications are conducted, were deformed. A protective sheath, called myelin, that normally wraps and boosts axons’ efficiency-- like insulation on an electrical wire – was reduced by one-third in the treated animals. This damage was three times worse in male than in female pups and would likely result in abnormal communication between the two hemispheres, say the researchers.
Moreover, the perinatally exposed animals showed evidence of neurons firing out of sync and other electrophysiological abnormalities, suggesting faulty organization of neuronal networks in the cortex.
The research also was supported by the NIH’s National Center for Research Resources, National Institute of Neurological Disorders and Stroke and National Institute of Child Health and Human Development.
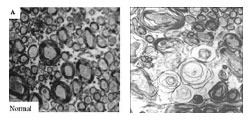
Cross-sections of the part of the rat brain that connects the left and right hemisphere (corpus collosum) show stunted development of neuronal wiring, called axons, in an animal that received an antidepressant (right) during a critical period around the time of birth. A protective sheath, called myelin (visible in normal animal at left), that normally wraps the axons and boosts their efficiency, failed to develop normally in the treated animal. The resultant inefficient neuronal communications could underlie the pattern of deficits seen in autism.
Source: Rick C.S. Lin, Ph.D., University of Mississippi Medical Center
Reference
Perinatal antidepressant exposure alters cortical network function in rodents. Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, Rodriguez-Porcel F, Paul IA, Merzenich M, Lin RC. Proc Natl Acad Sci U S A. 2011 Oct 24. [Epub ahead of print] PMID:22025710
***
The mission of the NIMH is to transform the understanding and treatment of mental illnesses through basic and clinical research, paving the way for prevention, recovery and cure. For more information, visit the NIMH website.
The National Center for Research Resources (NCRR), a part of NIH, provides laboratory scientists and clinical researchers with the resources and training they need to understand, detect, treat and prevent a wide range of diseases. NCRR supports all aspects of translational and clinical research, connecting researchers, patients and communities across the nation. NINDS is the nation's leading funder of research on the brain and nervous system. The NINDS mission is to reduce the burden of neurological disease — a burden borne by every age group, by every segment of society, by people all over the world.
About the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): The NICHD sponsors research on development, before and after birth; maternal, child, and family health; reproductive biology and population issues; and medical rehabilitation. For more information, visit the Institute’s Web site at http://www.nichd.nih.gov/ .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit the NIH website.
