Archived Content
The National Institute of Mental Health archives materials that are over 4 years old and no longer being updated. The content on this page is provided for historical reference purposes only and may not reflect current knowledge or information.
New Findings Reveal Surprising Role of the Cerebellum in Reward and Social Behaviors
NIH-funded study sheds new light on brain circuits related to affective and social dysfunction
• Press Release
A new study in rodents has shown that the brain’s cerebellum—known to play a role in motor coordination—also helps control the brain’s reward circuitry. Researchers found a direct neural connection from the cerebellum to the ventral tegmental area (VTA) of the brain, which is an area long known to be involved in reward processing and encoding. These findings, published in Science, demonstrate for the first time that the brain’s cerebellum plays a role in controlling reward and social preference behavior, and sheds new light on the brain circuits critical to the affective and social dysfunction seen across multiple psychiatric disorders. The research was funded by the National Institute of Mental Health (NIMH), part of the National Institutes of Health.
“This type of research is fundamental to deepening our understanding of how brain circuit activity relates to mental illnesses,” said Joshua A. Gordon, M.D., Ph.D., director of NIMH. “Findings like the ones described in this paper help us learn more about how the brain works, a key first step on the path towards developing new treatments.”
The cerebellum plays a well-recognized role in the coordination and regulation of motor activity. However, research has also suggested that this brain area contributes to a host of non-motor functions. For example, abnormalities in the cerebellum have been linked to autism, schizophrenia, and substance use disorders, and brain activation in the cerebellum has been linked to motivation, social and emotional behaviors, and reward learning, each of which can be disrupted in psychiatric disorders.
These earlier findings led Kamran Khodakhah, Ph.D., of the Albert Einstein College of Medicine, New York, and colleagues to wonder if there was a direct connection between the cerebellum and the VTA—a brain structure involved in controlling reward and motivational behaviors. To examine this, the researchers used a technique called optogenetics, in which the neurons of animals are genetically modified, so they can be controlled using pulses of light. The researchers used this technique in mice, activating neurons in the cerebellum which connected to the VTA. The researchers found that activating the cerebellar neurons led to increased activation in the VTA of mice, indicating a working connection between these two brain structures.
Once the neural connection between these two brain structures was confirmed, the researchers investigated whether inputs from the cerebellum to the VTA influenced reward-related and social preference behavior. The researchers placed mice in a square-shaped open chamber and used pulses of light to activate cerebellar neurons linked to the VTA whenever mice entered a specific part of the chamber, called the “reward quadrant.” Mice showed a strong preference for spending time in the reward quadrant, freely choosing to spend more than 70 percent of their time in this area. In addition, the researchers found that mice were willing to work for activation in this brain area and to spend time in conditions they would usually not prefer (light vs. darker areas) to receive this activation. Together, the findings suggest that activation of the cerebellar projections to the VTA is rewarding for mice and that the cerebellum plays a role in reward-related behaviors.
To examine whether the inputs from the cerebellum into the VTA impacted social behaviors, the researchers tested mice using a three-chamber social task in which the mice could choose to spend time in a chamber with another mouse (the social chamber), in an empty central chamber, or in a chamber containing a non-social object. At baseline, mice preferred to be in the social chamber, but after researchers inactivated the cerebellar projections into the VTA, the mice no longer showed this preference. In addition, continuous silencing of the cerebellar-VTA pathway was found to completely prevent the expression of social preference behavior in the mice, findings which indicate that inputs from the cerebellum into the VTA are necessary for social preference behavior in mice.
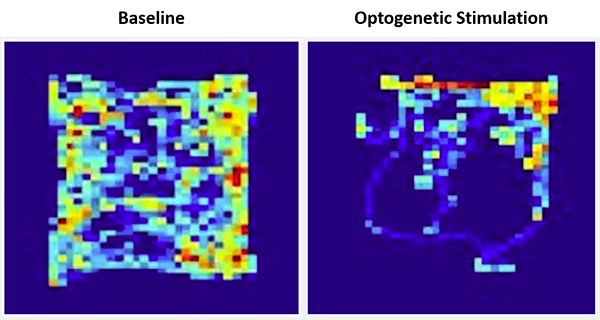
Heatmaps showing the amount of time a mouse spent in locations of the open field chamber at baseline (left) and during optogenetic stimulation of the cerebellar input to the VTA (right). The “reward quadrant” is located in the upper right section of the field. Credit: Albert Einstein College of Medicine
The results of this study suggest a potentially major—and previously unrecognized—role for the cerebellum in the creation and control of reward and social preference behaviors. Although there is much left to explore, the identification of this direct neural pathway may help explain the role of this circuit in disorders that involve reward-related and social-processing systems, such as addiction, autism, and schizophrenia, and may point to future targets for intervention and symptom management.
“The role of cerebellar circuitry in mental-health relevant behaviors is an understudied area, one in which we have just begun to see increased interest, said Janine Simmons, M.D., Ph.D., chief of the NIMH Social and Affective Neuroscience Program. “We are always excited to see innovation of this type in the behavioral neurosciences, and these results demonstrate how much remains to be learned.”
In future studies, the researchers plan to test whether the cerebellum-VTA pathway can be manipulated, using drugs or optogenetics, to treat addiction and prevent relapse after treatment.
“Cerebellar abnormalities are also linked to a number of other mental disorders such as schizophrenia,” said Dr. Khodakhah. “We want to find out whether this pathway also plays a role in those disorders.”
Reference
Carta, I., Chen, C. H., Schott, A., Dorizan, S., & Khodakhah, K. (in press). Cerebellar modulation of the reward circuitry and social behavior. Science. Online January 17, 2019.
Grants
MH115604 ; RR027888 ; NS050808 ; DA044761
This press release describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is foundational to advancing new and better ways to prevent, diagnose, and treat disease. Science is an unpredictable and incremental process— each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without the knowledge of fundamental basic research.
About the National Institute of Mental Health (NIMH): The mission of the NIMH is to transform the understanding and treatment of mental illnesses through basic and clinical research, paving the way for prevention, recovery and cure. For more information, visit the NIMH website.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit the NIH website .
NIH…Turning Discovery Into Health®
