2016 Spring Inside NIMH

Welcome
Welcome to the latest edition of Inside NIMH! We publish Inside NIMH in conjunction with each meeting of the National Advisory Mental Health Council, which advises the Secretary of Health and Human Services (HHS), the Director of the National Institutes of Health (NIH), and the Director of NIMH on all policies and activities relating to the conduct and support of mental health research, research training, and other programs of the Institute. In addition, check out our website for regular updates on timely topics at NIMH. I hope you find this information interesting and helpful. Please let us know if you have questions or comments on this Spring 2016 edition.
Sincerely,
Bruce Cuthbert, Ph.D.
Acting Director, National Institute of Mental Health
If you wish to unsubscribe, subscribe, or change your email address, please contact the NIMH Webmaster or visit the Inside NIMH subscription page.
Message from the NIMH Director
Many new activities are springing up at NIMH. The NIMH Director Search is ongoing, the National Advisory Mental Health Council (NAMHC) workgroups have been busy, NIH announced a change in AIDS/HIV research funding, the National Academies of Sciences hosted a discussion on the future of neuroscience clinical trials, the HHS Autism Coordinator was named, and new overtime provisions will affect postdoctoral researchers.
Spring Happenings
- The NIMH Director Search: Everyone at NIMH is eagerly awaiting word as to when the next NIMH Director will be named by Francis Collins, M.D., Ph.D., NIH Director, and when the new Director will start. The general understanding is that the search committee has tendered a short list of the finalists to Dr. Collins, and the NIH Office of the Director is working hard to reach a final decision. However, no definite word is available at press time.
- Updates from NAMHC Workgroups:
- One NAMHC workgroup is focused on Opportunities and Challenges of Developing Information Technologies on Behavioral and Social Science Clinical Research. This group has been charged with addressing how new mHealth technologies can be used to help achieve more objective and precise diagnosis and treatment of mental illnesses, and how such technologies can be used to help predict and prevent mental illnesses and improve the quality of mental health practice. On March 7, 2016, the workgroup held a meeting to review NIMH’s existing portfolio as it relates to the use of technology in the prevention, diagnosis, and treatment of mental illnesses. In addition, the group discussed the first three of five key questions related to this issue: 1) What technologies need to be developed to understand the life course and etiology of mental disorders in terms of their developmental trajectory, course, and epidemiology?; 2) How can these new technologies be used to predict and prevent mental illness?; and, 3) How can these new technologies be used to achieve more efficient and effective diagnoses and treatments of mental illnesses? At the next meeting on June 13, 2016, the workgroup will discuss the two remaining key questions: 4) How can these new technologies be used to improve quality in mental health practice?; and, 5) How can these new technologies enable new questions to be asked, and enable research to move more rapidly and to become more nimble?
- On April 5-6, 2016, the NAMHC convened a workgroup to develop a list of well-specified tasks, paradigms, and measures for investigators to consider using when conducting research involving the constructs of the Research Domain Criteria (RDoC) framework. Participants of the NAMHC Workgroup on Tasks and Measures for RDoC were invited from institutions throughout the United States and represented a wide variety of research expertise. At the end of the two-day meeting, the workgroup converged on tasks that met several desired criteria, such as reliability, validity, and ease of use for each construct. The workgroup is finalizing a report of the proceedings and a list of recommended tasks for inclusion in the RDoC Matrix for the NAMHC to consider.
- New NIH HIV/AIDS Research Priorities and Guidelines for Determining AIDS Funding: The NIH Office of AIDS Research (OAR) has announced a change in guidelines for determining whether a research project has a high-, medium-, or low-priority for receiving AIDS-designated funds beginning in fiscal year 2016. For a description of these priority topics and examples of each, see NOT-OD-15-137 . These guidelines do not assess/determine the scientific and technical merit of a project, but rather the priority of the research for receiving AIDS-designated funds. As a result, some research that does not completely target HIV/AIDS issues may be deemed a low priority for AIDS dollars. Applicants are encouraged to contact program staff in the NIMH Division of AIDS Research for guidance prior to submitting an application.
- Neuroscience Trials of the Future : Given the current challenges in clinical trials involving nervous system disorders, the Health and Medicine Division (formerly the Institute of Medicine (IOM)) of the National Academies of Sciences hosted a public workshop on March 3-4, 2016. This workshop brought together key stakeholders to discuss opportunities to improve the integrity, efficiency, and validity of clinical trials for nervous system disorders and mental illnesses, focusing specifically on Phase II and Phase III trials. Sarah Morris, Ph.D., Acting Director, NIMH RDoC Unit, presented “Diagnosis and Patient Identification: The RDoC Approach.” Dr. Morris shared examples of how heterogeneous symptoms and impairments (often reported by clinicians) support the need for an RDoC framework that looks at psychopathology without identifying a specific disorder. She also encouraged participants to use the RDoC Database (RDoCdb), an informatics platform for the sharing of human subjects data related to research on mental illnesses. RDoCdb integrates all data types (e.g., behavioral, imaging, and genetics), facilitates data sharing and data mining across studies, and is part of the larger NIMH Data Archive which has information from over 100,000 subjects.
- NIH Marijuana and Cannabinoids Neuroscience Research Summit: On March 22-23, 2016, NIH hosted a research summit focused on the neurological and psychiatric effects of marijuana, other cannabinoids, and the endocannabinoid system. Scientists from across the country presented their research, discussing both the adverse and the potential therapeutic effects of the cannabinoid system. The goal of the summit was to ensure evidence-based information is available to inform practice and policy, particularly important at this time given the rapidly shifting landscape regarding the recreational and medicinal use of marijuana. The meeting was sponsored by several NIH Institutes and Centers: NIMH; National Institute on Drug Abuse (NIDA); National Institute on Alcohol Abuse and Alcoholism (NIAAA); National Center for Complementary and Integrative Health (NCCIH); and, National Institute of Neurological Disorders and Stroke (NINDS).
- New HHS Autism Coordinator: Thomas Novotny, M.D., M.P.H., has been named as the Autism Coordinator and Deputy Assistant Secretary for Health (Science and Medicine), Office of the Assistant Secretary at HHS. Dr. Novotny attended the April 19, 2016 Interagency Autism Coordinating Committee (IACC) meeting. In his new role he hopes to complement the work of the IACC to coordinate research efforts among the different agencies and the work they do to support the seven priority aims of the IACC Strategic Plan . In addition, his office will be leading the effort to coordinate a cross-agency report concerning transition of youth with autism spectrum disorder to adulthood, as required in the Autism Collaboration, Accountability, Research, Education, and Support (CARES) Act. Dr. Novotny plans to focus on increasing acceptance, care, and support for individuals on the autism spectrum and their families, as outlined in his first blog post titled “HHS Embraces Autism Awareness and Acceptance: Improving Opportunities for Individuals with Autism and Their Families .”
- New Overtime Provisions Will Affect Postdoctoral Researchers: The Department of Labor has increased the overtime pay threshold to $47,476, effective December 1, 2016. This will impact NIH supported postdoctoral researchers, as described by Michael Lauer, M.D., NIH Deputy Director for Extramural Research in his "Open Mike" blog . NIH is determining how this rule will be implemented and how it will affect grantees, and will issue a notice in the NIH Guide in the coming months.
Budget Overview
- Fiscal Year (FY) 2016 Budget: NIMH anticipates awarding over 500 new and competing research project grants (RPGs) in FY 2016, with an estimated success rate of 21 percent, as can be seen in Figure 1 below. FY 2016 will be the fifth consecutive year that NIMH has exceeded 500 competing RPGs. Overall, as in past years, NIMH expects to support at least 75 percent of the applications up to the 20th percentile. Moreover, the Institute will give special consideration to applications from early stage investigators . With the exception of specific programmatic adjustments, NIMH will fully fund modular and non-modular grant awards. Future year commitments for modular grant awards are expected to remain consistent with the FY 2016 awarded amount. Future year commitments for competing non-modular grant awards will be reduced, on average, 10 percent from recommended funding levels and will not include increases for inflation in future years. Non-competing continuation awards in FY 2016 will be made at the committed level, and out-year commitments for continuation awards in FY 2017 and beyond will remain unchanged.
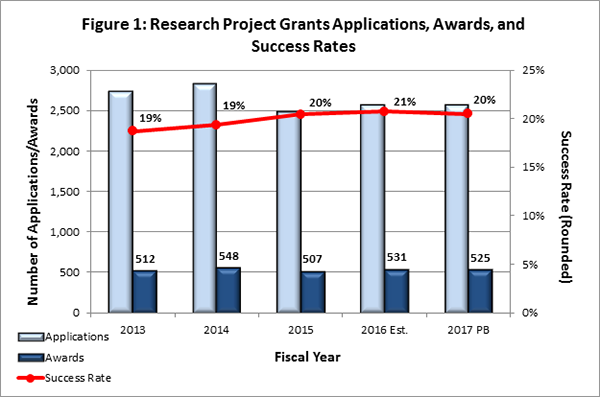
Presidents Budget (PB)
Figure 2 below shows the NIMH budget in appropriated (current) versus constant (FY 2000) dollars. Constant dollars are inflation adjusted for variations in the purchasing power of the dollar over time. Dollar amounts are adjusted based on the Biomedical Research and Development Price Index (BRDPI). The annual change in BRDPI indicates how much the NIH budget must change to maintain purchasing power similar to FY 2000.
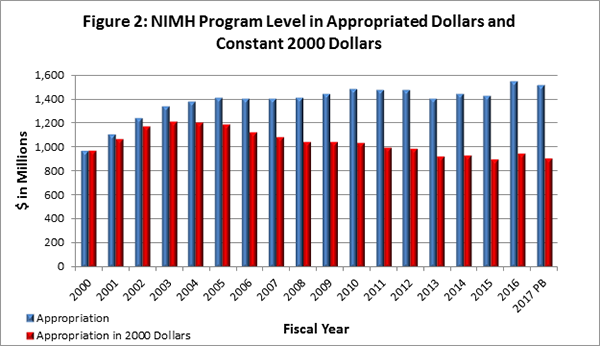
Presidents Budget (PB)
- Outlook for FY 2017: In February 2016, the President submitted his FY 2017 budget request to Congress. The request for NIH is $33.136 billion, an increase of $0.825 billion over the FY 2016 operating level. The request for NIMH is $1.5 billion, which is level with the FY 2016 budget. The FY 2017 NIH budget request increases BRAIN Initiative funding by $45 million, increases funding for the Precision Medicine Initiative by $100 million, and provides funding for the Cancer Moonshot at $680 million, for a total of $825 million.
Both House and Senate Appropriations Subcommittees held hearings with several NIH Institute and Center Directors. It is unclear when the Labor/HHS/Education appropriations bill, which contains the NIH appropriation, will be introduced. The Bipartisan Budget Act of 2015 increases the FY 2017 non-Defense discretionary sequester-level spending cap by approximately 3 percent. However, spending level determined by sequestration in the Budget Control Act of 2011 could still be instituted if there is no additional specific congressional action.
NIMH Staff News
- Meredith Fox, Ph.D., was named Acting Director of the NIMH Office of Science Policy, Planning, and Communications (OSPPC) effective April 5, 2016 and we are awaiting NIH approval on the final appointment. Dr. Fox received her Ph.D. in Behavioral Neuroscience/Psychology from American University. Following her Ph.D., she was appointed as an Assistant Professor in the Department of Psychology at American University. In 2005, Dr. Fox joined the Laboratory of Clinical Science in the NIMH Division of Intramural Research Programs where she studied serotonin and its transporter. In 2012, Dr. Fox joined the NIMH OSPPC, first via a detail and then as a Health Policy Analyst in the Science Policy and Evaluation Branch, rising to Branch Chief in 2014.
Director’s Highlights: NIMH Scientists and Science
Grantee Awards
NIMH is proud to recognize significant achievements and awards received by our current grantees:
- On February 18, 2016, President Obama announced the 2013 Presidential Early Career Awards for Scientists and Engineers (PECASE), the highest honor bestowed by the United States Government on science and engineering professionals in the early stages of their independent research careers. Three of the awardees are NIMH grantees:
- Kafui Dzirasa, M.D., Ph.D. (Duke University)
- Tina Goldstein, Ph.D. (University of Pittsburgh)
- Courtney Miller, Ph.D. (Scripps Florida)
- Sachin Patel, M.D., Ph.D. (Vanderbilt University)
- 2016 Marsha Linehan Award for Outstanding Research in the Treatment of Suicidal Behavior; American Association of Suicidology
- David A. Jobes, Ph.D. (Catholic University of America)
Notable NIMH Grants
The following is a selection of the Institute’s most recently funded projects that exemplify our efforts to accelerate research on mental illnesses and to advance the NIMH Strategic Plan for Research:
- Chronic stress and its effects are increasingly recognized as primary factors contributing to psychopathology in vulnerable children and adolescents. Preadolescence is a crucial time during which children’s ability to recognize stress and its causes matures, and is a time for building skills for managing stress. It is also a period of increased brain changes and growth in key self-regulatory physiological systems. Therefore, preadolescence might be an optimal time for intervening to prevent the onset of anxiety, depression, and post-traumatic stress symptoms in children faced with chronic stress. Martha Wadsworth, Ph.D. (Pennsylvania State University) aims to demonstrate that children facing chronic stress from exposure to poverty, discrimination, and violence can acquire and utilize new ways of coping. She and her team will also test whether these new coping skills have positive effects on stress physiology and changes in clinical symptoms. This exploratory evaluation of the Building a Strong Identity and Coping Skills (BaSICS) intervention is an early-stage, proof-of-concept study; if successful, it may yield preliminary data to support the development of an application for a confirmatory efficacy trial.
- Under two cooperative agreements, Diego Pizzagalli, Ph.D. (McLean Hospital) and Jared William Young, Ph.D. (University of California, San Diego) will form a consortium for sharing data and technical knowledge. The two research teams will attempt to identify cross-species translational measures to improve therapeutic development. Dr. Pizzagalli and his team aim to develop novel neurophysiological and behavioral assays of reward and cognition that are expected to be functionally similar across humans and rats. Dr. Young and his team aim to develop behavioral task parameters with accompanying biomarkers that quantify the behavioral domains related to cognitive control and motivation that can be tested in both humans and mice. Measures from these two studies are expected to improve the translational value of preclinical animal testing, which is a critical initial step for the development of much needed treatments for disorders of reward and cognition.
For more information on these and other grants selected for funding, please visit the NIH RePORTER website .
NIMH Science Updates
The latest news and updates from NIMH-supported research:
- World Leaders and Advocates Unite in Washington, D.C. for One Mission: Make Mental Health a Global Priority (May 20, 2016)
- NIMH Grantees Named Recipients of Prestigious Presidential Award (May 11, 2016)
- Secrets to Our Smarts Hidden in the Folds of Our Cortex (April 29, 2016)
- Biomarker Tracks Accelerated HIV-Associated Aging (April 21, 2016)
- Distractible Mice Offer Clues to Attention Deficit (March 24, 2016)
- Facebook Q&A on Electroconvulsive Therapy (March 17, 2016)
- RDoC Launches User-Friendly Matrix Format (March 7, 2016)
- Symptoms Outdo Diagnoses in Predicting Bipolar Disorder in At-Risk Youth (February 26, 2016)
- A BRIGHT Technological Future for Mental Health Trials (February 19, 2016)
- Psychophysiology: Special Issue Features RDoC Initiative (February 16, 2016)
Publicizing NIMH research is a communal responsibility. Please help us spread the word about the results of NIMH funding by acknowledging our support of your research, for example, in journal articles (citing your NIMH award by number when possible) and other communications. NIMH has two primary methods of getting the word out: press releases and science updates. All releases and updates are posted to the Science News section of the NIMH Web site. These are also distributed to the public through a mailing list .
Connect with NIMH
Inside NIMH is produced by the National Institute of Mental Health. For more information about the Institute, visit our website at https://www.nimh.nih.gov. For comments and suggestions about Inside NIMH, please contact the NIMH Webmaster. The material in this newsletter is not copyrighted, and we encourage its use or reprinting.
Our newest effort to reach our stakeholders is a service that allows you to subscribe for updates sent directly to your email inbox on the NIMH topics of your choice. In addition to our email newsletters and RSS updates, please also visit NIMH on Twitter , Facebook , and YouTube , where we highlight Science Updates, Press Releases, and other timely matters.
