2014 Winter Inside NIMH

Welcome to the latest edition of Inside NIMH. We publish Inside NIMH in conjunction with each meeting of the National Advisory Mental Health Council, which advises the Secretary of Health and Human Services, the Director of the National Institutes of Health, and the Director of NIMH on all policies and activities relating to the conduct and support of mental health research, research training, and other programs of the Institute. In addition, check out the NIMH Director’s Blog website for regular updates on timely topics at NIMH. I hope you find this information interesting and helpful. Please let us know if you have questions or comments on this edition.
Sincerely,
Tom Insel, MD
Director, National Institute of Mental Health
If you wish to unsubscribe, subscribe, or change your email address, please contact the NIMH Webmaster or visit the Inside NIMH subscription page .
Table of Contents
- Message from the NIMH Director
- Director’s Highlights: NIMH Scientists and Science
- New Announcements about Funding Opportunities
- Future Research Directions
- Update on Electronic Research Administration (eRA) Activities
- Director’s Blog
- NIMH Science Updates
- Connect with NIMH
I. Message from the NIMH Director
You may remember 2013 for the 16-day government shutdown and the 5.2 percent sequester. But don’t forget NIMH highlights for 2013, including the Nobel Prize in Physiology or Medicine awarded to NIMH grantee Thomas Südhof; advances such as CLARITY (Clear Lipid-exchanged Anatomically Rigid Imaging/immunostaining-compatible Tissue hYdrogel); the announcement of the BRAIN (Brain Research through Advancing Innovative Neurotechnologies) Initiative; and, the implementation of the Affordable Care Act, which includes a long-overdue policy change—mental health parity.
As we begin 2014, we look forward to reviewing the first round of BRAIN Initiative applications; to developing the Early Prediction and Prevention of Psychosis (EP3) initiative, which continues our efforts to improve outcomes for individuals at risk for or experiencing psychotic disorders; and, to refining the way that we conduct clinical trials, working to accelerate the pace of discovery through an experimental therapeutics approach to evaluating novel interventions for mental disorders.
New Priorities
-

- Developing methods for classifying and accessing the diverse cells and circuits of the brain (RFA-MH-14-215 ; RFA-MH-14-216 );
- Developing technologies for recording and modulating collections of cells that function together as a circuit (RFA-NS-14-007 ; RFA-NS-14-008 ; RFA-NS-14-009 ); and,
- Forming teams of scientists to develop the next generation of non-invasive imaging technologies for human research (RFA-MH-14-217 ).
- Enhancing the Reliability of NIMH-supported Research through Rigorous Study Design and Reporting: Recent industry studies have reported failures to replicate preclinical results from NIH-funded studies in cancer and other biomedical research areas. In response, NIMH issued a notice in the NIH Guide (NOT-14-004 ) which illustrates our commitment to the highest standards of rigor in both pre-clinical and clinical research. In addition to rigor, transparency is essential with detailed information made available about study design, execution, analysis, and interpretation. Examples of critical elements for a well-designed study are summarized in Enhancing the Reliability of NIMH-supported Research through Rigorous Study Design and Reporting on the NIMH website.
- New Focus for Clinical Trials: In addition to focusing on rigorous study design and reporting, we are working to transform our clinical trials portfolio by focusing on an experimental therapeutics approach. Both biomedical and psychosocial interventions will need to study targets or mediators as well as efficacy and safety. Clinical trials will need to meet new standards of efficiency, transparency, and data sharing. In Fiscal Year (FY) 2014, NIMH is planning to issue funding opportunity announcement (FOAs) to support several new mechanisms for clinical trials while withdrawing support from most omnibus clinical trial announcements (see related Concept Clearance in Section IV below).
- NeuroBioBank: In November 2013, NIMH, along with the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke, announced the NIH NeuroBioBank initiative, creating a new framework to collect, process, and store human brain tissue. Brains have been collected in the past, but by individual labs for individual disorders. The NIH NeuroBioBank is a strategy to collect tissue for many disorders and create a registry that will allow any scientist with a pressing question to have access to the tissue needed to find an answer. Data and tissue sharing have already transformed genomics. Sharing brain tissue is more complicated because, unlike DNA which can be replicated, the brain resource is not renewable.
Budget Overview
- FY 2013 Budget: NIMH awarded 512 new and competing research project grants (RPGs) in 2013 and achieved an overall success rate of 19 percent (defined as the number of RPG applications funded divided by the number of applications received; see Figures 1 and 2 (note that in Figure 2, the total number of funded grants do not add up to 512, as not all grants receive percentile scores). This represents a decrease of 72 awards below the 584 RPGs awarded in FY 2012, but is comparable with the FY 2009-2011 average of 522. NIMH awarded grants to 96 New Principal Investigators, and achieved a success rate of 22 percent for Early Stage Investigators (ESIs).
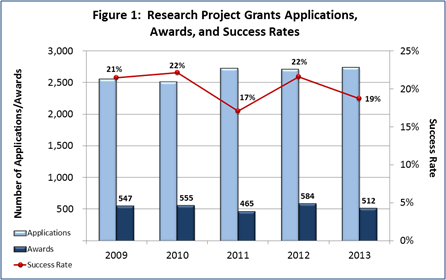
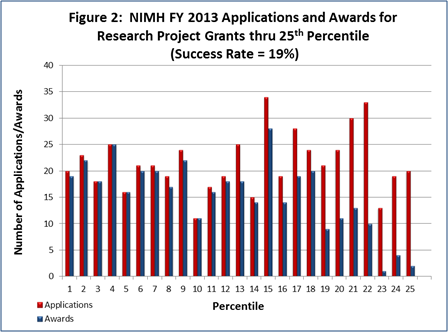
Figure 3 (below) shows the NIMH budget in appropriated (current) versus constant (FY 1998) dollars. Constant dollars are “inflation adjusted” for variations in the purchasing power of the dollar over time. Dollar amounts are adjusted based on the Biomedical Research and Development Price Index (BRDPI). The annual change in BRDPI indicates how much the NIH budget must change to maintain purchasing power similar to FY 1998.
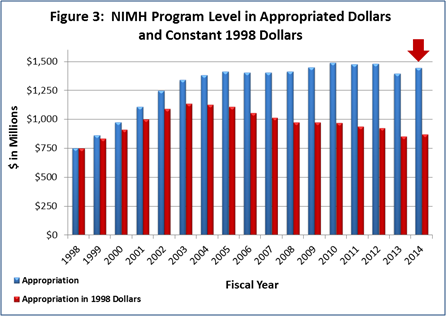
- FY 2014 Budget: On January 17, President Obama signed an omnibus appropriation (H.R. 3547) that funds NIH, along with most of the Federal government, through September 30, 2014. The amount provided to NIMH, $1.446 billion, represents a $50.2 million (3.6 percent) increase over the NIMH FY 2013 Budget Authority, but still a $32.7 million (2.2 percent) reduction below the FY 2012 Budget Authority. NIMH’s FY 2014 appropriation, along with several other NIH Institutes, includes a boost in funding intended for the President’s BRAIN Initiative .
Prior to the passing of the appropriation, NIH had been operating under a Continuing Resolution (CR). During the CR period, NIMH issued non-competing research grant awards at a level below that indicated on the most recent Notices of Award (generally up to 90 percent of the previously committed level). As with previous CRs, we look forward to upward adjustments after our grant funding policy is determined.
NIMH Program Updates
-

- The RAISE Early Treatment Program (ETP) is a large randomized controlled trial testing the effectiveness of a multicomponent intervention to reduce the symptoms of psychosis and prevent the gradual deterioration of functioning that is characteristic of chronic schizophrenia. Recruitment for the study ended in July 2012, with 403 individuals from 34 clinics across 21 states enrolled. Collection of two-year outcome data for study participants will conclude in June 2014.
- The RAISE Connection Program (RCP) is an implementation study, exploring ways to effectively integrate comprehensive, multicomponent treatment programs for first episode psychosis into existing state mental health systems. Through a program evaluation of two RCP clinics established in New York City and Baltimore, investigators have documented what is needed to implement key aspects of team-based early psychosis interventions in community settings. Based on the success of the RCP clinics, several programs were recently established:
- The New York State Office of Mental Health launched OnTrackNY , an evidence-based team approach to providing recovery-oriented treatment to young people who have recently begun experiencing psychotic symptoms.
- Four clinics were established in the Downstate New York region in 2013.
- The Maryland Mental Hygiene Administration announced the Center for Excellence on Early Intervention for Serious Mental Illness and has committed FY 2013 funds to establish four early psychosis clinics across the state.
- In August 2013, NIMH awarded additional support to the RCP to identify factors that may contribute to the duration of untreated psychosis among individuals who sought care in RAISE-associated specialty clinics, and develop community-based interventions to decrease time from symptom onset to treatment engagement.
- Early Prediction and Prevention of Psychosis (EP3): Continuing efforts to improve outcomes for individuals at risk for or experiencing psychotic disorders, NIMH recently launched the EP3 initiative. EP3 aims to support accelerated research on the detection of risk states for psychotic disorders, preventing onset of psychosis in high-risk subjects, and reducing the duration of untreated psychosis in people who have experienced a first psychotic episode. Several new FOAs have already been announced as part of this larger effort. These initial announcements are focused on high priority issues for the Institute:
- Research to Improve the Care of Persons at Clinical High Risk for Psychotic Disorders (RFA-MH-14-210 ; RFA-MH-14-211 ; RFA-MH-14-212 );
- FOAs released in April 2013 (PAR-13-187 ; PAR-13-188 ) yielded four funded studies which focus on reducing the period of untreated psychosis to less than 12 weeks from the currently estimated overall average in the US of 110 weeks. A fifth study on reducing the duration of untreated psychosis in communities with specialty care clinics for early psychosis was funded via a modification to the RAISE Connection Program contract.
NIMH Staff and Campus News
- New NIMH Staff and Staff Changes:
- Janet Clark, PhD, joined the NIMH Division of Intramural Research Programs (IRP) in October 2013 as the Director of the Office of Fellowship Training. Prior to joining the NIMH IRP, Janet served as Associate Professor in the Department of Pharmacology and Physiology; Director of the Pharmacology and Physiology Graduate Program; and Co-director of the Drug Discovery and Development Program at Drexel University College of Medicine. Janet is charged with the development and oversight of the NIMH IRP's integrated multidisciplinary training program and with providing support and resources for all training-related activities.
- Amy Goldstein, PhD, joined NIMH in November 2006 and serves as Chief of the Child and Adolescent Preventive Intervention Research Program of the Division of Services and Intervention Research. In October 2013, Amy was named as the NIMH Associate Director for Prevention. In this congressionally mandated position, she collaborates with other NIMH Divisions, including the IRP, other NIH Institutes, other federal agencies, and external organizations to build and manage an NIMH-wide, integrated prevention research program. Amy also represents NIMH on interagency and interdepartmental policy and program development initiatives in prevention.
- Jennifer Mehren, PhD, will join the NIMH IRP on January 27, 2014, in the role of Scientific Advisor. Jennifer comes to NIMH from the National Eye Institute, Office of Program Planning and Analysis, where she worked primarily on strategic planning for the Institute and program management for the trans-NIH Nanomedicine Initiative. At NIMH, Jennifer will manage the BSC reviews and will participate in IRP strategic planning and the annual review process.
- Maryland Pao, MD, was appointed as the Deputy Scientific Director of the NIMH IRP in December 2013. Maryland will continue in her role as the Clinical Director of the NIMH IRP, and also serves as Chief of the Psychiatry Consultation Liaison Service in the NIH Hatfield Clinical Research Center.
- Retirements:
- Sandy Markey, PhD, recently retired from his position as Chief of the Laboratory of Neurotoxicology in the NIMH IRP. Sandy joined NIMH in 1974 and developed a laboratory to investigate the metabolism of small molecules important in brain biochemistry. An international expert in gas chromatographic-mass spectrometric methods, his laboratory has focused on studying proteins in sub-cellular structures, particularly those relevant to neuronal development and synaptic function. In recent years, Sandy led NIH efforts in proteomics.
- Dennis Murphy, MD, recently retired from his position as Chief of the Laboratory of Clinical Science (LCS) in the NIMH IRP. Dennis joined NIMH as a clinical fellow in 1966 and became Chief of the Clinical Neuropharmacology Branch in 1977, which then was incorporated within LCS in 1983.While at NIMH, he explored the neurobiology of neuropsychiatric disorders using molecular, neurochemical, and genetic techniques in animals and humans, with a focus on obsessive compulsive disorder (OCD) and the serotonin transporter (SERT). In addition to publishing over 900 papers and four books, Dennis’ legacy includes training over 100 students and fellows, of whom more than 30 have served subsequently as chairs of psychiatry and basic science departments.
- Molly Oliveri, PhD, retired from her position as Director of the NIMH Division of Developmental Translational Research (DDTR). Following a career as a member of the research faculty at the George Washington University School of Medicine, Molly joined NIMH in 1987 as Chief of the Personality and Emotion Program, later becoming Chief of the Behavioral Science Research Branch. In 2004, she became Deputy Director, and in 2007 Director, of DDTR. As a Division Director, Molly stimulated the growth of integrative research in neurobehavioral mechanisms and trajectories of development and in novel mechanistic interventions for developmental psychopathology. She also served as a skilled leader of NIMH priority-setting activities and trans-NIH planning efforts to develop a lifespan human connectome.
- NIMH IRP Principal Investigator Awards:
- Jay N. Giedd, MD, Chief, Unit on Brain Imaging, Child Psychiatry Branch, NIMH, was honored with a Brain & Behavior Research Foundation Award.
- Daniel S. Pine, MD, Chief, Emotion and Development Branch, NIMH, was elected to be a member of the Institute of Medicine (IOM) of the National Academies.
-

Return to Top
II. Director’s Highlights: NIMH Scientists and Science
Grantee Awards
NIMH is proud to recognize significant awards received by our current grantees:
-

- Thomas Südhof, MD, Avram Goldstein Professor of Molecular and Cellular Physiology and Howard Hughes Medical Institute Investigator, Departments of Neurology, Psychiatry, and Behavioral Sciences, Stanford University. Along with his co-awardees Dr. Randy Schekman and Dr. James Rothman, Dr. Südhof was recognized for his discoveries on vesicle trafficking. Dr. Südhof’s work identified key molecules involved in the release of neurotransmitters from vesicles in the presynaptic terminal, and revealed the molecular mechanisms that trigger the very rapid signaling between neurons that underlie brain computational processing. In addition, Dr. Südhof has made important contributions in the field of synapse formation, describing how precise connections emerge in the CNS and how these connections might be compromised in complex brain disorders such as autism.
- The Institute of Medicine (IOM) of the National Academies:
- Ann E. Kurth, PhD, Professor and Executive Director, College of Nursing Global Division; Associate Dean for Research, Global Institute of Public Health.
- Pat R. Levitt, PhD, Provost Professor of Pediatrics, Neuroscience Psychiatry, and Pharmacy, WM Keck Chair in Neurogenetics, Keck School of Medicine, University of Southern California; Director, Program in Developmental Neurogenetics, Children's Hospital Los Angeles.
- Eve Marder, PhD, Head, Division of Science and Victor and Gwendolyn Benfield Professor of Neuroscience, Biology Department and Volen Center, Brandeis University.
- Louis J. Muglia, MD, PhD, Director, Center for Prevention of Preterm Birth, Cincinnati Children's Hospital Medical Center; Professor of Pediatrics, University of Cincinnati College of Medicine.
- Pamela Sklar, MD, PhD, Professor, Departments of Psychiatry, Neuroscience, and Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai.
- Matthew W. State, MD, PhD, Oberndorf Family Distinguished Professor and Chair, Department of Psychiatry, University of California, San Francisco.
- Christopher A. Walsh, MD, PhD, Investigator, Howard Hughes Medical Institute; Chief, Division of Genetics and Genomics, Boston Children's Hospital; Bullard Professor of Pediatrics and Neurology, Harvard Medical School.
- 1st Annual Mathematical Neuroscience Prize:
- Laurence F. Abbott, PhD, Co-director of the Center for Theoretical Neuroscience, Professor of Neurobiology and Behavior, Columbia University College of Physicians and Surgeons.
- Brain & Behavior Research Foundation Awards:
- Boris Birmaher, MD, Director, Child and Adolescent Anxiety Program, University of Pittsburgh Schools of the Health Sciences; Co-director, Child and Adolescent Bipolar Services, Western Psychiatric Institute and Clinic of University of Pittsburgh Medical Center.
- Marc G. Caron, PhD, James B. Duke Professor of Cell Biology, Duke University Medical Center.
- Karl Deisseroth, MD, PhD, D.H. Chen Professor of Bioengineering, and of Psychiatry and Behavioral Sciences, Stanford University.
- Kafui Dzirasa, MD, PhD, Assistant Professor, Psychiatry and Behavioral Sciences, Division of Geriatric Psychiatry, Duke University School of Medicine.
- Presidential Early Career Award in Science and Engineering (PECASE):
- Damien Fair, PhD, Assistant Professor, Behavioral Neuroscience and Psychiatry, Oregon Health and Science University.
Notable NIMH Grants
Here is a selection of the Institute’s most recently funded projects that exemplify our efforts to accelerate mental health research and to advance the NIMH Strategic Plan:
- One of the basic assumptions of genetic theory—that all cells of an individual have the same DNA sequence—may be an oversimplification. Called somatic mosaicism, this variability in DNA sequence from cell to cell may play an important role in brain development and in shaping an individual’s susceptibility and resilience to neuropsychiatric disorders. As little is known about the extent of somatic mosaicism in the normal human brain, Flora Vaccarino, MD , (Yale University) is investigating the degree of somatic variation in the developing human brain. These studies aim to improve our understanding of normal brain development, and may set the stage for understanding genetic diversity within the brain and its effect on the development of neuropsychiatric disorders.
- Research shows that the brain changes dramatically over childhood and adolescence, and two-thirds of mental disorders begin prior to the age of 21. However, the development of critical tools and databases for monitoring and evaluating normative brain functional maturation are necessary to further our understanding of the onset and progression of mental disorders. Michael Milham, MD, PhD , (Child Mind Institute, Nathan S. Kline Institute for Psychiatric Research) is generating a large set of multi-modal brain imaging data which will establish normative measures of brain function across childhood and adolescence, similar to growth curves used by pediatricians. Importantly, these data will be immediately shared with the broader scientific community, providing an open access resource and enabling research on the early detection of pathologic process before clinically significant symptoms appear.
- For individuals with serious mental illness (SMI), arrest and incarceration often delay or prevent receipt of mental health treatment services. Michael Compton, MD , (Lenox Hill Hospital)is developing and testing a jail diversion strategy whereby previously incarcerated adults with SMI who commit minor infractions can be redirected from jail to mental health services. Using this new police-mental health linkage system, police officers contact a mental health specialist for assistance in determining the best resolution for the individual and the situation. The ultimate goals are to ensure treatment continuity and to maintain a place in the community for people with SMI who are at risk for repeated incarcerations.
- Cognitive impairment occurs in up to 60 percent of depressed older adults and is one of the most debilitating and costly aspects of late-life depression. New Investigator Award recipient Robert Mackin, PhD , (University of California, San Francisco) is identifying the impact of several neurobiological characteristics (e.g., cerebral blood flow, cortical atrophy, and amyloid deposition) on cognitive decline and dementia in older adults with major depression, and determining the impact of depression on the course of cognitive decline in older adults. These studies are an add-on component of the Alzheimer's Disease Neuroimaging Initiative study (ADNI-II) funded by the National Institute on Aging and the Foundation for the NIH. Understanding the neurobiological characteristics of cognitive impairment provides an opportunity to improve health and disability outcomes for older adults with depression.
For more information on these and other grants selected for funding, please visit the NIH RePORTER website .
Return to Top
III. New Announcements about Funding Opportunities
Each week, NIH electronically distributes the NIH GUIDE , a listing of all NIH Funding Opportunity Announcements (FOAs) that include requests for applications (RFAs), program announcements (PAs), and important notices for the scientific community. Below is a selection of recently issued FOAs in which NIMH participates. The Funding page on the NIMH website has links to listings of all NIMH FOAs and other resources.
Note: You can subscribe to the NIMH Funding Opportunities ListServ to receive the latest information about RFAs and other research funding opportunities from NIMH, as well as administrative updates and changes to grant policies and procedures. You can also subscribe to a separate listserv to receive weekly emails of the NIH GUIDE .
NIMH-administered Requests for Applications
- Disaster Mental Health Research Intervention Center
- Release date: September 20, 2013; Expiration date: February 3, 2014
- P60 announcement (RFA-MH-14-090 )
- Exceptional Unconventional Research Enabling Knowledge Acceleration (EUREKA) for Neuroscience and Disorders of the Nervous System
- Release date: November 8, 2013; Expiration date: February 3, 2014
- R01 announcement (RFA-MH-14-214 )
- BRAIN Initiative: Transformative Approaches for Cell-Type Classification in the Brain
- Release date: December 17, 2013; Expiration date: March 13, 2014
- U01 announcement (RFA-MH-14-215 )
- BRAIN Initiative: Development and Validation of Novel Tools to Analyze Cell-Specific and Circuit-Specific Processes in the Brain
- Release date: December 17, 2013; Expiration date: March 13, 2014
- U01 announcement (RFA-MH-14-216 )
- BRAIN Initiative: Planning for Next Generation Human Brain Imaging
- Release date: December 17, 2013; Expiration date: March 13, 2014
- R24 announcement (RFA-MH-14-217 )
- Limited Competition to Develop a BrainSpan RNA-seq Browser
- Release date: January 15, 2014; Expiration date: March 28, 2014
- R24 announcement (RFA-MH-15-100 )
- National Cooperative Reprogrammed Cell Research Groups (NCRCRG) to Study Mental Illness
- Release date: May 8, 2013; Expiration date: January 8, 2016
- U19 announcement (PAR-13-225 )
- Reducing the Duration of Untreated Psychosis in the United States
- Release date: April 10, 2013; Expiration date: May 8, 2016
- R01 announcement (PAR-13-187 )
- R34 announcement (PAR-13-188 )
NIMH-collaborative Requests for Applications
- NIH Director's Early Independence Awards
- Release date: August 14, 2013; Expiration date: January 31, 2014
- DP5 announcement (RFA-RM-13-009 )
- Computational Analyses Exploiting Reference Epigenomic Maps
- Release date: December 10, 2013; Expiration date: March 3, 2014
- R01 announcement (RFA-RM-14-001 )
- BD2K-LINCS-Perturbation Data Coordination and Integration Center (DCIC)
- Release date: December 4, 2013; Expiration date: March 19, 2014
- U54 announcement (RFA-HG-14-001 )
- BRAIN Initiative: New Technologies and Novel Approaches for Large-Scale Recording and Modulation in the Nervous System
- Release date: December 17, 2013; Expiration date: March 24, 2014
- U01 announcement (RFA-NS-14-007 )
- BRAIN Initiative: Optimization of Transformative Technologies for Large Scale Recording and Modulation in the Nervous System
- Release date: December 17, 2013; Expiration date: March 24, 2014
- U01 announcement (RFA-NS-14-008 )
- BRAIN Initiative: Integrated Approaches to Understanding Circuit Function in the Nervous System
- Release date: December 17, 2013; Expiration date: March 24, 2014
- U01 announcement (RFA-NS-14-009 )
- NIH Director’s Biomedical Research Workforce Innovation Award: Broadening Experiences in Scientific Training (BEST)
- Release date: January 17, 2014; Expiration date: March 31, 2014
- DP7 announcement (RFA-RM-13-019 )
- Mentored Career Development Award in Biomedical Big Data Science for Clinicians and Doctorally Prepared Scientists
- Release date: January 15, 2014; Expiration date: April 1, 2014
- K01 announcement (RFA-HG-14-007 )
- Courses for Skills Development in Biomedical Big Data Science
- Release date: January 16, 2014; Expiration date: April 1, 2014
- R25 announcement (RFA-HG-14-008 )
- Open Educational Resources for Biomedical Big Data
- Release date: January 16, 2014; Expiration date: April 1, 2014
- R25 announcement (RFA-HG-14-009 )
- NIH Coordination and Evaluation Center for Enhancing the Diversity of the NIH-Funded Workforce Program
- Release date: December 19, 2013; Expiration date: April 2, 2014
- U54 announcement (RFA-RM-13-015 )
- NIH Building Infrastructure Leading to Diversity (BUILD) Initiative
- Release date: December 19, 2013; Expiration date: April 2, 2014
- U54 announcement (RFA-RM-13-016 )
- NIH National Research Mentoring Network (NRMN)
- Release date: December 19, 2013; Expiration date: April 2, 2014
- U54 announcement (RFA-RM-13-017 )
- Validation and Advanced Development of Technologies for the Study of Biological Properties of Single Cells
- Release date: December 19, 2013; Expiration date: April 4, 2014
- R33 announcement (RFA-RM-13-020 )
- Exceptionally Innovative Tools and Technologies for Single Cell Analysis
- Release date: December 19, 2013; Expiration date: April 4, 2014
- R21 announcement (RFA-RM-13-021 )
- Revisions to Add Single Cell Analysis to Active Research Projects
- Release date: December 19, 2013; Expiration date: April 4, 2014
- R01 announcement (RFA-RM-13-022 )
- U01 announcement (RFA-RM-13-023 )
- U.S.-South Africa Program for Collaborative Biomedical Research
- Release date: December 13, 2013; Expiration date: April 22, 2014
- R01 announcement (RFA-AI-14-009 )
- R21 announcement (RFA-AI-14-010 )
- NIH Pioneer Award Program
- Release date: August 8, 2013; Expiration date: Future dates: October 10, 2014; October 9, 2015
- DP1 announcement (RFA-RM-13-006 )
- NIH Director's New Innovator Award Program
- Release date: August 8, 2013; Expiration date: Future dates: October 17, 2014; October 16, 2015
- DP2 announcement (RFA-RM-13-007 )
- Development of Highly Innovative Tools and Technology for Analysis of Single Cells (SBIR)
- Release date: March 13, 2013; Expiration date: January 8, 2016
- R43/R44 announcement (PA-13-140 )
Return to Top
IV. Future Research Directions
Concept Clearances for Potential New Research Initiatives
This listing of potential future initiatives is meant to provide the earliest possible alert to the field of our research interests and of potential upcoming announcements to solicit that research. While NIMH plans to proceed with these initiatives, their publication and timing are not certain and depend on sufficient funding. The titles and brief descriptions are consistent with the information available at the time of concept clearance. The resultant FOAs may differ from the concepts in the final wording of their titles or other aspects. To send questions about a specific concept, follow the "Submit Comments" link at the bottom of the description.
- NIMH’s New Focus in Clinical Trials: The goal of this effort is to transform the manner in which NIMH solicits and conducts clinical trial research, and to focus applications towards an emphasis on the experimental therapeutics approach to the treatment and prevention of mental disorders in adults and children.
For more information, please see recent NAMHC-approved concepts, recent public venue-approved concepts, and past NAMHC meetings, which also contains links to meeting agendas, minutes, and Inside NIMH (Director’s Reports).
NIMH-sponsored Meetings
- Closing the Gaps: Reducing Disparities in Mental Health Treatment through Engagement: In September 2013, the NIMH Office for Research on Disparities and Global Mental Health hosted a meeting, “Closing the Gaps: Scaling Up to Reduce Mental Health Disparities in the United States.” The purpose of the meeting was to address the significant disparities in mental health care related to race and ethnicity. NIMH brought together leading experts in disparities research and practice from the public and private sectors to address specific questions and present relevant research findings. Participants included representatives from within the NIH community, other federal agencies, and researchers charged with improving the Nation’s mental health equity.
Return to Top
V. Update on Electronic Research Administration (eRA) Activities
Electronic Grant Application Submission News
- Multi-project Activity Codes Moving to Electronic Submission
- Additional multi-project activity codes are moving to electronic submission for due dates on or after January 25, 2014 (NOT-OD-13-075 ). A set of FAQs for submitting multi-project electronic applications through Application Submission System & Interface for Submission Tracking (ASSIST) is available.
- FORMS-C Application Package
- The new FORMS-C application packages have a different format and include new Planned Enrollment Report and Cumulative Inclusion Enrollment forms. These forms allow NIH to collect the data in a format that can be leveraged throughout the lifecycle of the application/grant.
- The forms are included in the application packages as 'Optional,' and eRA systems no longer provide an error when inclusion data are omitted. However, the policies on when to include the data in your application have not changed. Applicants must carefully follow the application guide and supplemental instructions to ensure the new forms are included when needed. Please see the Decision Tree for Monitoring Inclusion Based on Sex/Gender, Race, and Ethnicity in Research .
- Career Development (K), Fellowship (F), and Training (T and D) Programs Moving to FORMS-C
- A reminder notice regarding the transition of K, F, T, and D programs to updated electronic application forms has been posted (NOT-OD-14-027 ). These changes apply to submissions with due dates on or after January 25, 2014.
- K and T Parent Announcements were reissued on December 19, 2013, under new FOA numbers. All other active K, T, and D FOAs have been or will be updated to include a FORMS-C package with the existing announcement (i.e., no FOA number change). NIH has been making every effort to have the new FOAs and packages in place 45-60 days prior to the first due date that falls on or after January 25, 2014. F Parent Announcements will be reissued in time for the April 2014 due dates.
- New eRA Website
- The eRA website has been updated, and can be found at http://era.nih.gov .
- New Features in eRA Commons
- Research Progress Performance Report (RPPR) Open Pilot to All Federal Demonstration Partnership (FDP) Institutions: The RPPR in eRA Commons will accommodate submission of non-SNAP awards, including multi-project (complex) and training awards, as part of a pilot open to members of the FDP (see NOT-OD-13-113 ).
- Biomedical Workforce Initiative Tied to RPPR: Data from the new Personal Profile is used as part of the NIH initiative to track the biomedical research workforce so that informed decisions can be made about training of the optimal number of people for the appropriate types of positions that will advance science and promote health. As part of the validation process for RPPR submissions, eRA Commons will provide warnings when information on the Personal Profile is missing from key personnel identified in the report.
- Super Storm Sandy Reporting Link Now On the Status Screen: If you received special administrative supplement funding as a result of the effects of Super Storm Sandy, there is now a link in the eRA Commons Status screen to allow grantees to submitquarterly progress reports to a new reporting site and view these submissions.
- eRA Commons No Longer Accepts Full Social Security Numbers: To protect your information, the Personal Profile or any other data fields in eRA Commons no longer accepts a full Social Security Number.
- NIH Review Roster URL Has Changed: The public roster webpage "NIH Scientific Review Group (SRG) Roster Index" that hosts the rosters for NIH review meetings has moved to a new, more secure site that has been developed by eRA. The old URL, http://era.nih.gov/roster, has been changed to http://public.era.nih.gov/pubroster .
- Additional information can be found in the eRA Commons Release Notes .
- New Resources
- Reviewers: How to Set Up and Maintain Personal Profile in eRA Commons (YouTube, December 12, 2013)
- xTrain External User Guide (PDF)
- eRA Commons Online Help System
- Reviewers: Getting Reimbursement of Expenses & Honoraria (YouTube, November 26, 2013)
For more information on all of these updates, please see the NIH eRA News and Events page .
Questions? Contact the eRA help desk . Note that contacting this help desk is the only way to document problems with an electronic grant application submission. Evidence of this contact is the only way to be eligible for any special consideration by the Center for Scientific Review (CSR) Division of Receipt and Referral, should you run into a system problem with Grants.gov or with eRA that is beyond your control.
Return to Top
VI. Director’s Blog
The following blogs by former NIMH Director Thomas Insel are no longer available.
- Thinking about 2014 (January 7, 2014): Dr. Insel looks forward to what’s ahead in 2014 for neuroscience and mental health: both challenges and promise.
- Ten Best of 2013 (December 13, 2013): Dr. Insel reviews his “top ten” selections for 2013, including research advances and historic policy changes affecting mental health care.
- Wanted: A Few Good Brains (November 27, 2013): The importance of post-mortem brain donation to research and how the new NIH NeuroBioBank initiative will support research on human brain tissue.
- Culture Clash (November 22, 2013): The need for research taking place in academic settings to be in line with the desire of the public for advances in prevention and treatment of disease.
- P4C : Time = Lives (November 14, 2013): A recap of the annual Partners for Cure (P4C) meeting, where the theme is to speed the delivery of new cures through innovative research models.
- One Person, Many Genomes (November 6, 2013): The discovery that mutations unique to an individual are common in the brain both changes and complicates the search for genes underlying brain disorders.
- Shutdown (October 22, 2013): The short- and long-term effects of the shutdown.
Return to Top
VII. NIMH Science Updates
The latest news and updates from NIMH-supported research:
- Join the Drug Facts Chat Day (January 13, 2014)
- Transgenic Mice Lines Aid in Brain Circuit Imaging (December 18, 2013)
- NIMH Twitter Chat on Depression and Older Adults (December 11, 2013)
- NIH Directors Discuss Sequestration and Research on C-SPAN (December 6, 2013)
- NeuroBioBank Gives Researchers One-stop Access to Post-mortem Brains (November 27, 2013)
- Earliest Marker for Autism Found in Young Infants (November 6, 2013)
- NIMH Twitter Chat on Bullying Prevention (October 23, 2013)
- NIMH Scientists Honored with 2013 Brain & Behavior Research Foundation Awards (October 22, 2013)
- Streamlined Method Offers Shortcut to Generating Neurons for Discovery (October 21, 2013)
- Three NIH Scientists Elected into IOM (October 21, 2013)
- Exposure/Ritual Prevention Therapy Boosts Antidepressant Treatment of OCD (October 21, 2013)
- NIMH Grantee Receives 2013 Nobel Prize (October 17, 2013)
- Science/AAAS Google+ Hangout on the Adolescent Brain Featuring NIMH’s Jay Giedd, MD (September 27, 2013)
- Former NIMH Grantee Receives 2013 MacArthur Fellow Award (September 27, 2013)
- Jay Giedd on PBS Documentary “Brains on Trial” (September 19, 2013)
Publicizing NIMH research is a communal responsibility. Please help us spread the word about the results of NIMH funding by acknowledging our support of your research, for example, in journal articles (citing your NIMH award by number when possible) and other communications. NIMH has two primary methods of getting the word out: press releases and science updates. All releases and updates are posted to the Science News section of the NIMH Web site. These are also distributed to the public through a mailing list .
If you have a manuscript accepted for publication that describes an especially significant finding, please contact your NIMH Program Official to discuss the possibility of a news release or other forms of dissemination.
Return to Top
VIII. Connect with NIMH
Our newest effort to reach our stakeholders is a service that allows you to to subscribe for updates sent directly to your email inbox on the NIMH topics of your choice. In addition to our email newsletters and RSS updates, NIMH offers a vodcast series entitled “Speaking of Science,” and its own YouTube videos on mental health topics. We have also entered the world of Twitter and Facebook , where we highlight Science Updates, Press Releases, and other timely matters.
Inside NIMH is produced by the National Institute of Mental Health. For more information about the Institute, visit our Web site at http://www.nimh.nih.gov. For comments and suggestions about Inside NIMH, please contact the NIMH Webmaster. The material in this newsletter is not copyrighted, and we encourage its use or reprinting.
