Archived Content
The National Institute of Mental Health archives materials that are over 4 years old and no longer being updated. The content on this page is provided for historical reference purposes only and may not reflect current knowledge or information.
Ketamine Reverses Neural Changes Underlying Depression-Related Behaviors in Mice
NIH-supported study sheds light on the neural mechanisms underlying remission of depression
• Press Release
Researchers have identified ketamine-induced brain-related changes that are responsible for maintaining the remission of behaviors related to depression in mice – findings that may help researchers develop interventions that promote lasting remission of depression in humans. The study, funded by the National Institute of Mental Health (NIMH), part of the National Institutes of Health, appears in the journal Science.
“Ketamine is a potentially transformative treatment for depression, but one of the major challenges associated with this drug is sustaining recovery after the initial treatment,” said study author Conor Liston, M.D., Ph.D ., of Weill Cornell Medicine, New York City.
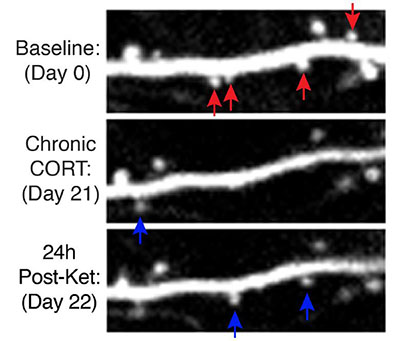
To understand mechanisms underlying the transition from active depression to remission in humans, the researchers examined behaviors related to depression in mice. Researchers took high-resolution images of dendritic spines in the prefrontal cortex of mice before and after they experienced a stressor. Dendritic spines are protrusions in the part of neurons that receive communication input from other neurons. The researchers found that mice displaying behaviors related to depression had increased elimination of, and decreased formation of, dendritic spines in their prefrontal cortex compared with mice not exposed to a stressor. This finding replicates prior studies linking the emergence of behaviors related to depression in mice with dendritic spine loss.
In addition to the effects on dendritic spines, stress reduced the functional connectivity and simultaneous activity of neurons in the prefrontal cortex of mice. This reduction in connectivity and activity was associated with behaviors related to depression in response to stressors. Liston’s group then found that ketamine treatment rapidly restored functional connectivity and ensemble activity of neurons and eliminated behaviors related to depression.
Twenty-four hours after receiving a single dose of ketamine, mice exposed to stress showed a reversal of behaviors related to depression and an increase in dendritic spine formation when compared to stressed mice that had not received ketamine. These new dendritic spines were functional, creating working connections with other neurons.
The researchers found that while behavioral changes and changes in neural activity in mice happened quickly (three hours after ketamine treatment), dendritic spine formation happened more slowly (12-24 after hours after ketamine treatment). While further research is needed, the authors suggest these findings might indicate that dendritic spine regrowth may be a consequence of ketamine-induced rescue of prefrontal cortex circuit activity.
Although dendritic spines were not found to underly the fast-acting effects of ketamine on behaviors related to depression in mice, they were found to play an important role in maintaining the remission of those behaviors. Using a new technology developed by Haruo Kasai, M.D., Ph.D., and Haruhiko Bito, Ph.D., collaborators at the University of Tokyo, the researchers found that selectively deleting these newly formed dendritic spines led to the re-emergence of behaviors related to depression.
“Our results suggest that interventions aimed at enhancing synapse formation and prolonging their survival could be useful for maintaining the antidepressant effects of ketamine in the days and weeks after treatment,” said Dr. Liston.
“Ketamine is the first new anti-depressant medication with a novel mechanism of action since the 1980s. Its ability to rapidly decrease suicidal thoughts is already a fundamental breakthrough,” said Janine Simmons, M.D., Ph.D., chief of the NIMH Social and Affective Neuroscience Program. “Additional insights into ketamine’s longer-term effects on brain circuits could guide future advances in the management of mood disorders.”
Reference:
Moda-Sava, R. N., Murdock, M. H., Parekh, P. K., Fetcho, R. N., Huang, B. S., Huynh, T. H., … & Liston, C. (in press). Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science.
Grants:
MH097822 ; MH109685 ; MH118451
About the National Institute of Mental Health (NIMH): The mission of the NIMH is to transform the understanding and treatment of mental illnesses through basic and clinical research, paving the way for prevention, recovery and cure. For more information, visit the NIMH website.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit the NIH website .
NIH…Turning Discovery Into Health®
