Archived Content
The National Institute of Mental Health archives materials that are over 4 years old and no longer being updated. The content on this page is provided for historical reference purposes only and may not reflect current knowledge or information.
Patient-Derived Support Cells Stunt Mouse Brain Development
Errant glia may underlie childhood-onset schizophrenia illness process
• Science Update
At least some cases of schizophrenia may be caused by an illness process rooted in wayward support cells instead of the neurons they sustain, suggest experiments by NIMH-funded researchers. Such glial cells, generated – via a disease-in-a-dish technology – from patients with childhood onset schizophrenia, stunted neural circuit development when grafted into developing mouse brains. The animals grew up to display anxiety-like behaviors, antisocial tendencies, sleep-disturbances, and a lack of motivation, mimicking some features of the human illness.
NIMH grantee Steven Goldman, M.D., Ph.D., of the University of Rochester, and colleagues, report on their findings in the August 3, 2017 issue of Cell Stem Cell. An accompanying editorial heralds their discovery as “one of the most creative and compelling uses of stem cell technology for disease modeling” – with potential implications for improved treatments.
Although evidence was mounting of glial-related abnormalities in schizophrenia prior to the new study, researchers didn’t know whether these might just be secondary to a neuron-rooted illness process. Animal models to sort this out were lacking, so Goldman and his team set out to develop one. They generated induced pluripotent stem cells (iPSCs) from skin cells of patients who had experienced onset of psychosis before puberty. While rare, such cases of childhood onset schizophrenia are thought to be more genetically-influenced and severe than more typical cases with onset in late adolescence or early adulthood.
The researchers, who included Drs. Paul Tesar and Robert Findling of Case Western University, then induced the iPSCs – which can potentially differentiate into any kind of tissue – to develop into glial cells, which play a pivotal supporting role in brain development. Once in the developing mouse brain, these cells out-competed the animal’s own glia, resulting in adult mice with mouse neurons and human glia. This allowed comparisons of glial function from unaffected people and those with schizophrenia – but in a living, behaving organism instead of in a dish.
The result: The schizophrenia-affected brains didn’t mature sufficiently and failed to create a proper network to support neurons. Stunted growth of astrocytes, a type of glial cell, stymied development of intercellular connections. Another glial type, oligodendrocytes, failed to adequately sheath neurons with signal-conducting myelin – a deficiency also commonly seen in individuals with schizophrenia. Expression of 118 genes was disrupted, including some on the “skyline” of chromosomal sites previously implicated in genome-wide studies of schizophrenia risk.
Strikingly, transplanting iPSC glia derived from skin cells of individuals without schizophrenia into mouse brains resulted in no such adverse effects on brain circuitry or the behavioral and cognitive deficits reminiscent of schizophrenia.
“The results suggest a strong causal contribution of glial pathology to schizophrenia,” said Goldman.
“This is exciting work, both in terms of the surprising finding and the potential for this type of analysis to yield insight into how schizophrenia disrupts complex brain function,” added Dr. David Panchision, chief of the NIMH Developmental Neurobiology Program, which supports the study. “It will potentially allow researchers to biologically distinguish the disease progression in patients who develop schizophrenia early versus later in life, so that more targeted therapies can be developed.”
For More Information
Faulty support cells disrupt communication in brains of people with schizophrenia
U. Rochester release
https://www.eurekalert.org/pub_releases/2017-07/uorm-fsc071717.php
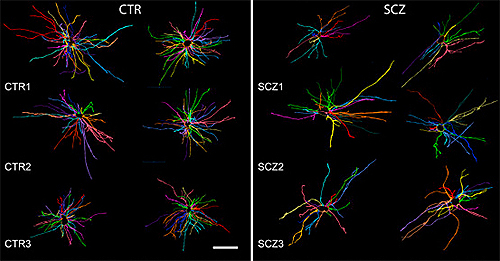
onset schizophrenia (right) showed signs of stunted growth compared to
those from normal controls (left).
Source: Steven Goldman, M.D., Ph.D., University of Rochester
References
Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Windrem MS, Osipovitch M, Liu Z, Bates J, Chandler-Militello D, Zou L, Munir J, Schanz S, McCoy K, Miller RH, Wang S, Nedergaard M, Findling RL, Tesar PJ, Goldman SA. Cell Stem Cell. 2017 Aug 3;21(2):195-208.e6. doi: 10.1016/j.stem.2017.06.012. Epub 2017 Jul 20. PMID: 28736215
Do Not Adjust Your Mind: The Fault Is in Your Glia. Franklin RJM, Bullmore ET. Cell Stem Cell. 2017 Aug 3;21(2):155-156. doi: 10.1016/j.stem.2017.07.005. PMID: 28777939
Grants
MH099578, MH104701, NS75345
