2015 Autumn Inside NIMH

Welcome to the latest edition of Inside NIMH. We publish Inside NIMH in conjunction with each meeting of the National Advisory Mental Health Council, which advises the Secretary of Health and Human Services (HHS), the Director of the National Institutes of Health (NIH), and the Director of NIMH on all policies and activities relating to the conduct and support of mental health research, research training, and other programs of the Institute. In addition, check out the NIMH Director's Blog for regular updates on timely topics at NIMH. I hope you find this information interesting and helpful. Please let us know if you have questions or comments on this edition.
Sincerely,
Tom Insel, MD
Director, National Institute of Mental Health
If you wish to unsubscribe, subscribe, or change your email address, please contact the NIMH Webmaster or visit the Inside NIMH subscription page.
Message from the NIMH Director
Unlike the leaves of the forest when autumn hath blown, a host of fresh developments awaits in this newsletter.
Policy and Portfolio
- Autism Biomarkers Consortium: Government, non-profit, and other private partners are funding a multi-year project to develop and improve clinical research tools for studying autism spectrum disorder (ASD). The project will receive a total of $28 million over the next four years to test and refine clinical measures of social impairment in ASD in order to better evaluate potential behavioral and drug therapies. The Consortium is supported by NIH (specifically, NIMH, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development), the Foundation for the NIH, the Simons Foundation Autism Research Initiative, and others. The effort is the latest addition to the prestigious list of projects supported by the Biomarkers Consortium , a large public-private partnership that aims to accelerate biomedical research progress. The Consortium supports research to identify disease-specific biomarkers and develop targeted technologies and treatments, with the ultimate goal of precision medicine.
- Precision Medicine Initiative (PMI): Over the last few months, NIH has held a series of workshops across the country to gather input from participant, scientific, and other stakeholder groups as it plans the development of the PMI and the vision for building the million-person national research cohort. Most recently, NIH convened a meeting to discuss digital health data in the cohort, a workshop on participant engagement and health equity , and a workshop on mobile and personal technologies . Here at NIMH, the Institute’s Research Domain Criteria (RDoC) initiative is designed to help bring precision medicine to mental illnesses. Just as NIH has invited input into the NIH-wide PMI, NIMH continues to invite the research community to debate, discuss, and enhance the RDoC concept via the RDoC Discussion Forum.
- Update on the BRAIN InitiativeSM : On June 3-4, 2015, NIH convened a meeting of stakeholders and interested parties to disseminate information on opportunities for research using latest-generation devices for neuromodulation and interfacing with the brain in humans. NIH presented a framework for facilitating and lowering the cost of new studies using these devices. Participants discussed regulatory and intellectual property considerations, and provided recommendations for data coordination and access. Following the meeting, NIH released a Request for Information entitled, BRAIN Initiative Industry Partnerships for Early Access to Neuromodulation and Recording Devices for Human Clinical Studies (NOT-NS-15-032 ). See the BRAIN Initiative webpage for video recordings of the meeting and other background information. For more information about NIMH-led BRAIN Initiative funding announcements, please contact BRAIN-info-NIMH@mail.nih.gov.
- Common Rule Notice of Proposed Rulemaking (NPRM): HHS and 15 other Federal Departments and Agencies have announced proposed revisions to modernize, strengthen, and make more effective the Federal Policy for the Protection of Human Subjects that was promulgated as a Common Rule in 1991. An NPRM was published on September 8, 2015 by the Office of the Federal Register for a 90-day public comment period (see also this HHS press release ). The NPRM seeks comment on proposals to better protect human subjects involved in research, while facilitating valuable research and reducing burden, delay, and ambiguity for investigators. There are plans to release several webinars that will explain the changes proposed in the NPRM, and a town hall meeting planned to be held in Washington, D.C. in October.
- Institute of Medicine (IOM) Report on Psychosocial Interventions: On July 14, 2015, the IOM released a report entitled, Psychosocial Interventions for Mental and Substance Use Disorders: A Framework for Establishing Evidence-Based Standards . The report developed from a meeting the IOM had convened to identify key steps to ensure that evidence-based, high-quality care is provided to individuals receiving mental health and substance use services. As described in the report, the resulting framework aims to incorporate the consumer’s perspective in order to improve the outcomes of psychosocial interventions. The framework emphasizes: (1) research on the efficacy and effectiveness of psychosocial interventions; (2) key elements of improved health outcomes; (3) systematic reviews to inform clinical guidelines; (4) quality measures of the structures, processes, and outcomes of interventions; and (5) methods for successfully implementing, sustaining, and improving psychosocial interventions in regular practice.
- Clinical Trials Portfolio: In a recently published white paper entitled, “Portfolio, Progress to Date, and the Road Forward,” NIMH Associate Director for Clinical Research Nitin Gogtay, MD and I reviewed NIMH’s clinical trials portfolio and the steps the Institute has taken to change how the portfolio is funded and managed. The paper outlines three calls for change to address the current slow pace and high cost of treatment for mental illnesses, and how NIMH is answering that call: improved efficiency, increased transparency, and adequate monitoring of human-subjects protection and privacy issues. NIMH has implemented a number of new requirements for clinical trials, and NIMH Program Officers stand ready and willing to help investigators to define trial milestones, and they will carefully monitor the initiation, recruitment, and completion of each trial. Continued funding will be contingent upon meeting milestones. NIMH will also consider a history of efficient performance in making funding decisions for new trials. In addition, in recognition of the need to focus more on target validation and experimental therapeutics, NIMH clinical trials now require testing for target engagement as a potential mechanism of disease.
Budget Overview
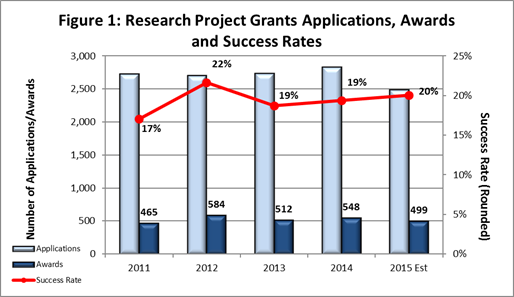
- Fiscal Year (FY) 2015 Budget: As shown in Figure 1, we are currently on track to award approximately 499 new and competing research project grants (RPGs) in FY 2015, a decrease from the 525 we estimated for this year. The reduction is primarily due to a higher average cost per competing award. However, due to a lower than anticipated number of competing RPG applications received this year, the projected success rate for competing RPGs in FY 2015 is 20%, an increase from the FY 2014 success rate of 19%. The projected success rate for Early Stage Investigators is 23%, similar to the rate for FY 2014. We anticipate funding a total of 105 new Principal Investigators (PIs) in FY 2014, compared to the 96 new PIs we funded in FY 2014.
- Outlook for FY 2016: FY 2016 will almost certainly begin under a continuing resolution (CR). As in the past, while operating under a CR, non-competing grants will be awarded at levels below committed amounts, likely at 90%. As in previous years, when we operate under a CR, the commitment level for NIMH grants will be determined after we receive a full-year appropriation for FY 2016.
NIMH Staff News and Awards
- Philip Wang, MD, DrPH has left his position as NIMH Deputy Director to join the American Psychiatric Association as Director of Research, effective August 21, 2015. Dr. Wang has served as the Deputy Director for NIMH since 2009, during which time he has served the Institute in myriad ways, from assisting in the oversight of over 1,000 NIMH staff and a budget of $1.4 billion, to spearheading major initiatives and partnerships with other federal agencies aimed at helping pave the way for the prevention, recovery, and cure of mental illnesses. Dr. Wang joined NIMH in 2006 as the Director of the Division of Services and Intervention Research, bringing his formidable knowledge of psychiatry, epidemiology, and mental health care systems. For the past year, Dr. Wang has also served the role of Acting Director for the Division of Translational Research. Sarah Hollingsworth Lisanby, MD will be joining NIMH as Director of the Division on October 1, 2015. Kathleen Anderson, PhD, the current Deputy Director, is leading the Division effective August 24, until Dr. Lisanby arrives. In addition, Bruce Cuthbert, PhD, the Director of the NIMH RDoC Unit, has agreed to take on the responsibility of NIMH Acting Deputy Director.
- As noted in the Spring 2015 edition of Inside NIMH, Thomas Lehner, PhD, the Director of the NIMH Office for Genomics Research Coordination (OGRC), has been appointed as the Senior Genomics Advisor in the Office of the Scientific Director, NIMH Division of Intramural Research Programs. With this new role, Dr. Lehner will be able to better oversee the strategic directions of genomic research for the entire Institute. Therefore, Dr. Lehner has relinquished his duties as Chief for the Genomics Research Branch in the Division of Neuroscience and Basic Behavioral Science, which he had led since 2006. Anjene Addington, PhD has agreed to serve as Acting Chief, effective July 20, 2015, while a national search is ongoing. In addition, Geetha Senthil, PhD will become the Program Official for all grants in the OGRC, effective October 1, 2015.
- NIMH is pleased to announce that Jean Noronha, PhD, current Acting Director for the Division of Extramural Activities (DEA), will become the permanent Director pending official NIH approval. Dr. Noronha is a graduate of Loyola University, where she earned her doctorate in Biochemisty and Biophysics from the Stritch School of Medicine. She joined NIMH in 1989 as a scientific review officer from the intramural research program at the National Institute of Aging where she was a senior staff fellow. Over the years, Dr. Noronha has taken on increasing responsibilities, first as the Institute’s Acting Review Branch Chief, then as the Chief Extramural Policy and Referral Liaison, and, since 2009, as the Deputy Director for DEA. We congratulate her on this appointment.
- The NIMH Division of Intramural Research Programs (IRP) is pleased to announce the promotion of Zheng Li, PhD (Chief, Unit on Synapse Development and Plasticity) to tenured status, and the promotion of Armin Raznahan, MD, PhD (named as a Lasker Clinical Research Scholar earlier this year) as a tenure-track investigator (Chief of the Developmental Neurogenomics Unit). The IRP is also pleased to wish autumn welcomes to new tenured investigator Yogita Chudasama, PhD (joining as the Chief of the Section on Behavioral Neuroscience, as well as Director of the Rodent Behavioral Core), as well as Mario Penzo, PhD, who will join the IRP as a tenure-track investigator (Chief of the Unit on the Neurobiology of Affective Memory).
- Joel Sherrill, PhD, Program Official in the Division of Services and Intervention Research, has received a Meritorious Research Service Commendation from the American Psychological Association (APA) Board of Scientific Affairs. The commendation, which will be presented at the December 2015 meeting of APA’s Board of Directors, recognizes Dr. Sherrill’s leadership contributions at NIMH and acknowledges his efforts at identifying research priorities for federal funding, designing funding initiatives, supporting the implementation of research projects, and nurturing the careers of junior and mid-career scientists.
- In Memoriam: We are saddened at the passing of Louis (Lou) Sokoloff, MD on July 30, 2015. Dr. Sokoloff joined the Institute in 1953, in the Laboratory of Neurochemistry. Taking the research lead for the Section on Cerebral Metabolism, which would eventually become the Laboratory of Cerebral Metabolism in 1957, Dr. Sokoloff did the foundational work for modern PET imaging, studying the physiological and biochemical processes involved with brain metabolism, and how this, in turn, was related to functional activity. His groundbreaking work developing the 2-deoxyglucose method resulted in numerous awards and widespread recognition, including election to the National Academy of Sciences in 1980; the Albert Lasker Clinical Medical Research Award in 1981; the Karl Spencer Lashley Award in 1987; and, along with his mentor, Seymour Kety, MD, NIMH’s first Scientific Director, the National Academy of Sciences Award in the Neurosciences in 1988. Although Dr. Sokoloff officially retired from NIMH in 2004, he continued to be an active presence on campus, working in an office in the Laboratory of Neuropsychology.
Director’s Highlights: NIMH Scientists and Science
Grantee Awards
NIMH is proud to recognize significant achievement and awards received by our current grantees:
- American Psychological Association, Distinguished Scientific Contribution Award:
- Edna Foa, PhD (University of Pennsylvania)
- Gruber Foundation Prize:
- Carla Shatz, PhD (Stanford University)
- Michael Greenberg, PhD (Harvard Medical School)
- Klingenstein-Simons Fellowship Awards in the Neurosciences:
- Michael Halassa, MD, PhD (New York University School of Medicine)
- Byungkook Lim, PhD (University of California, San Diego)
Notable NIMH Grants
Here is a selection of the Institute’s most recently funded projects that exemplify our efforts to accelerate mental health research and to advance the NIMH Strategic Plan for Research:
- Children with ASD experience persistent deficits in social communication and social interaction early in life. Researchers have been stymied in their investigation of the causes of social impairment in ASD, due in part to a lack of sensitive and objective methods to measure social function. James McPartland, PhD (Yale University) and collaborators at Duke University, Boston Children’s Hospital, University of California at Los Angeles, and University of Washington – all members of the Autism Biomarkers Consortium noted above – have tackled this challenge by developing a set of reliable behavioral and psychophysiological tools for use in clinical trials. The research team aims to compare lab-based measures of domains of social impairment to commonly used, standardized clinician and caregiver assessments of social function. The team also aims to evaluate the utility of eye-tracking responses and measures of brain activity via electroencephalogram as biomarkers for future clinical trials. The research will lay the groundwork for ASD researchers to select valid subgroups of children and reliably measure the clinical effects of interventions.
- Even effective and innovative treatments for mental illnesses can be hamstrung if individuals are reluctant to acknowledge their illness and/or seek help. Reducing stigma associated with mental illness may increase the likelihood an individual will seek and obtain care and benefit from it, but there is insufficient evidence regarding how stigma reduction campaigns can best achieve those aims. A new study led by Rebecca Collins, PhD (RAND Corporation) will employ existing real-world data collection systems to identify strategies for improving access, quality, and equity of mental health services in diverse populations in California. The study will determine whether a state-wide, multi-component social marketing campaign aimed at reducing stigma and discrimination associated with mental illnesses will also contribute to increased treatment-seeking and improved functional and social outcomes for people with a mental illness. The mass-media marketing campaign will be supplemented by websites, toolkits, outreach efforts, and educational training, and is expected to reach millions of people.
- Parents’ lifetime exposures to stress, infection, malnutrition, and advanced age have been linked with an increased risk for neurodevelopmental disorders in children. While maternal insults during pregnancy have been shown to directly affect fetal development, the mechanisms by which paternal lifelong experiences can affect offspring neurodevelopment are not well understood. Using a rodent model of stress experience in males, Tracy Bale, PhD (University of Pennsylvania) aims to explore mechanisms by which stress-induced changes in sperm microRNAs result in neurodevelopment changes to HPA stress axis regulation, blood-brain barrier permeability, and transmitted epigenetic changes in microRNA in offspring, and how such changes give rise to offspring who exhibit increased stress reactivity.
For more information on these and other grants selected for funding, please visit the NIH RePORTER website .
Director’s Blog
The NIMH Director’s Blog provides insights into the latest topics in mental health research:
The following blogs are no longer available.
- Look Who is Getting into Mental Health Research (August 31, 2015): Tech companies are bringing their ability to extract knowledge from data to health care. These examples show the potential of new tech-based approaches to diagnosis and treatment.
- August at NIMH (August 21, 2015): Despite its reputation as a month for slowing down, August is busy at NIMH as the end of the fiscal year approaches. Dr. Insel takes time out to give an update on NIMH-supported clinical trials.
- The Brain’s Critical Balance (July 29, 2015): The BRAIN Initiative is supporting scientists aiming to understand how the 86 billion neurons in the brain act together to enable consciousness and behavior.
- Quality Counts (July 14, 2015): The Institute of Medicine has issued a report looking at the effectiveness of psychosocial treatments for mental disorders. It is essential that consumers needing treatment receive evidence-based therapies.
- Viewing the STARRS Data (July 9, 2015): June 30 marked the end of the first phase of Army STARRS; July 1 marked the release of Army STARRS data for use by the broad scientific community.
- Accentuate the Positive: Rhythm and Blues (June 30, 2015): Researchers were able to reverse some of the behavioral effects of stress in mice by stimulating brain cells activated by pleasure, with implications for understanding depression.
- Early BRAIN Breakthroughs (June 22, 2015): Read about recent breakthroughs from the BRAIN Initiative, which show the promise of what we can accomplish with investment focused on new tool development to better understand and treat brain disorders.
- Something Interesting is Happening (June 5, 2015): The Precision Medicine Initiative will create a new kind of patient-driven research, which is similar to how innovative companies have created a new share economy based on trust.
- Training for the Future (May 26, 2015): Incorporating neuroscience in the training of psychiatric residents is important, and a new initiative has been designed to do that. The clinician of 2025 will need to know about the science of the brain.
NIMH Science Updates
The latest news and updates from NIMH-supported research:
- Psychosis Treatment Program Expands in New York (August 26, 2015)
- Webinar Series – Office for Research on Disparities and Global Mental Health (August 12, 2015)
- Attention-Control Video Game Curbs Combat Vets’ PTSD Symptoms (July 24, 2015)
- NIH Joins Public-Private Partnership to fund Research on Autism Biomarkers (July 16, 2015)
- NIH Joins with Women’s Organization to Debut Postpartum Depression Video (July 16, 2015)
- Boys More Likely to Have Antipsychotics Prescribed, Regardless of Age (July 1, 2015)
- Study May Help Department of Veterans Affairs Find Patients with High Risk of Suicide (June 11, 2015)
- A Patient’s Budding Cortex – in a Dish? (May 29, 2015)
Publicizing NIMH research is a communal responsibility. Please help us spread the word about the results of NIMH funding by acknowledging our support of your research, for example, in journal articles (citing your NIMH award by number when possible) and other communications. NIMH has two primary methods of getting the word out: press releases and science updates. All releases and updates are posted to the Science News section of the NIMH Web site. These are also distributed to the public through a mailing list .
Connect with NIMH
Inside NIMH is produced by the National Institute of Mental Health. For more information about the Institute, visit our website at https://www.nimh.nih.gov. For comments and suggestions about Inside NIMH, please contact the NIMH Webmaster. The material in this newsletter is not copyrighted, and we encourage its use or reprinting.
Our newest effort to reach our stakeholders is a service that allows you to subscribe for updates sent directly to your email inbox on the NIMH topics of your choice. In addition to our email newsletters and RSS updates, NIMH offers a vodcast series entitled “Speaking of Science” and YouTube videos on mental health topics. Please also visit NIMH on Twitter and Facebook , where we highlight Science Updates, Press Releases, and other timely matters.
