2016 Winter Inside NIMH

Message from the NIMH Director
Welcome to the latest edition of Inside NIMH! We publish Inside NIMH in conjunction with each meeting of the National Advisory Mental Health Council, which advises the Secretary of Health and Human Services (HHS), the Director of the National Institutes of Health (NIH), and the Director of NIMH on all policies and activities relating to the conduct and support of mental health research, research training, and other programs of the Institute. In addition, check out our website for regular updates on timely topics at NIMH. I hope you find this information interesting and helpful. Please let us know if you have questions or comments on this edition.
Sincerely,
Bruce Cuthbert, PhD
Acting Director, National Institute of Mental Health
If you wish to unsubscribe, subscribe, or change your e-mail address, please contact the NIMH Webmaster or visit the Inside NIMH subscription page.
As we settle into the New Year, we have an opportunity to reflect on some recent changes at NIMH. As you are likely aware, after 13 years of service as the Director of NIMH, Thomas Insel, MD, bid us farewell and moved on to Verily (formerly Google Life Sciences). During this transition period as we search for the new NIMH Director, I am honored to serve as the Acting Director of NIMH; in this role, I hope to strengthen the Institute while maintaining the normal execution of operations to advance the NIMH mission. In addition to these and other changes at NIMH, there is a lot going on at NIH, including a new children’s research initiative, an increase in support for the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative , and the release of the NIH-Wide Strategic Plan .
What’s New & What’s News
- NIMH Director Search: A national search for the new NIMH Director is underway. Walter Koroshetz, MD, Director of the National Institute of Neurological Disorders and Stroke, and Nora Volkow, MD, Director of the National Institute on Drug Abuse, are co-chairing the search committee. Applications are due by February 8, 2016.
- The National Advisory Mental Health Council (NAMHC) Workgroup on The Opportunities and Challenges of Developing Information Technologies on Behavioral and Social Science Clinical Research: The NAMHC, which advises NIMH, has formed a new workgroup focused on Behavioral and Social Science Research at NIMH. On November 24, 2015, the workgroup held an initial planning meeting. The workgroup has been charged with addressing how new mHealth technologies can be used to help achieve more objective and precise diagnosis and treatment of mental illnesses, and how such technologies can be used to help predict and prevent mental illnesses and improve the quality of mental health practice. In pursing this charge, the workgroup will approach the question of how we can develop and use technology-driven information science at several levels of analysis, including the individual (cognition and behavior), social, and cultural contexts.
- Institute of Medicine (IOM) Report on Evidence-Based Standards for Psychosocial Interventions for Mental Disorders : The IOM convened a workgroup in early 2014 to address issues about the evaluation of psychosocial interventions, including such topics as training providers in evidence-based therapies and determining the fidelity with which treatments are delivered. The workgroup’s report, released in July, 2015, detailed reasons for the gap between known effective treatments compared to current typical practice, and outlined a framework for providing standards for psychosocial interventions. A follow-up conference was held in October, 2015 to discuss the implementation of the workgroup’s recommendations. NIMH has already implemented research projects to study efficient strategies for employing evidence-based therapies and effective processes for improving quality, and a potential initiative focused on Pragmatic Strategies for Assessing Psychotherapy Quality in Practice was presented at the May 29, 2015 meeting of the NAMHC.
- The New Interagency Autism Coordinating Committee (IACC): The IACC, newly re-formed under the Autism Collaboration, Accountability, Research, Education, and Support (CARES) Act, met in November, 2015 and again in January, 2016 with the Acting Director of NIMH, Dr. Bruce Cuthbert, serving as Chair of the IACC. The IACC is an advisory committee that offers guidance to the HHS Secretary and works across federal agencies while coordinating with the autism community. Topics discussed at the meetings included developing an updated IACC Strategic Plan for Autism Spectrum Disorder (ASD) Research , and preparing an annual summary of advances in ASD research for 2014 and 2015. Under the new CARES Act, the committee will expand its focus on services and supports, while continuing its work on advancing autism research.
- BRAIN Initiative :
- Funding Opportunities: In the recent omnibus spending bill passed on December 18, 2015, Congress continued to strongly support the BRAIN Initiative. The bill provides $150 million for the BRAIN Initiative, an increase of $85 million above fiscal year (FY) 2015, to be pooled from various NIH Institutes and Centers. This news followed the October 1, 2015, NIH announcement of the second wave of grants to support the goals of the BRAIN Initiative, bringing the NIH investment to $85 million in fiscal year (FY) 2015. The latest round of projects will focus on visualizing the brain in action.
- BRAIN Initiative Investigator’s Meeting: The second annual meeting to discuss the BRAIN Initiative was held in December, 2015. Breakout sessions focused on cell type histology and morphology, next generation human non-invasive neuroimaging, and neural recording and modulation technologies. NIMH grantee Karl Deisseroth, MD, PhD (Stanford University, Howard Hughes Medical Institute), gave the plenary address Optical Tools for Discovery in Neuroscience .
- Environmental Influences on Child Health Outcomes (ECHO) Program: NIH has launched a new seven-year initiative, called ECHO, to better understand the effects of environmental exposures on child health and development. The goals of ECHO are consistent with those of the former National Children’s Study, and will capitalize on existing participant populations. ECHO is designed to support approaches that can evolve with the science and take advantage of technological advances and the growing number of clinical research networks. Neurodevelopment is one of the key pediatric outcomes with high public health impact that these studies will examine. The NIH awarded $144 million in new grants in FY 2015 to support ECHO and the development of new tools to enhance measurement of environmental exposures. For more information on ECHO funding opportunity announcements , see the recently hosted NIH webinars .
- NIH-Wide Strategic Plan: On December 16, 2015, NIH released the NIH-Wide Strategic Plan, Fiscal Years 2016–2020: Turning Discovery Into Health . As requested by Congress, the plan outlines a vision for biomedical research to capitalize on new opportunities for scientific exploration and to address new challenges for human health. Developed with input from hundreds of stakeholders and scientific advisers, and in collaboration with leadership and staff of NIH’s Institutes, Centers, and Offices (ICOs), the plan is designed to complement the strategic plan of each ICO, which are aligned with their congressionally mandated missions. NIMH published our Strategic Plan for Research in March, 2015.
Budget Overview
- Fiscal Year (FY) 2015 Budget: NIMH awarded 507 new and competing research project grants (RPGs) in 2015, and achieved an overall success rate of 20 percent (defined as number of RPG applications funded divided by the number of applications received; see Figures 1 and 2 (note that in Figure 2, the total number of funded grants do not add up to 507, as not all grants are percentiled)). NIMH awarded grants to 83 new Principal Investigators, and achieved a success rate of 23 percent for Early Stage Investigators .
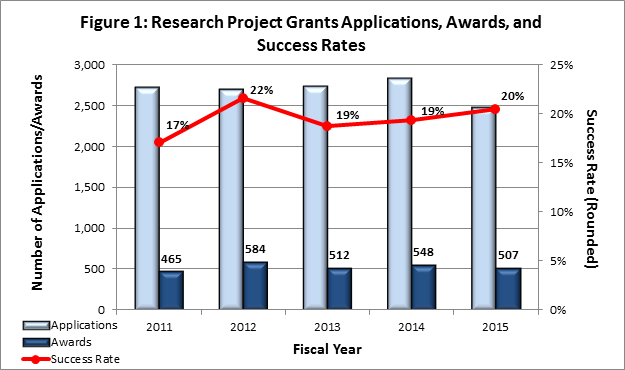
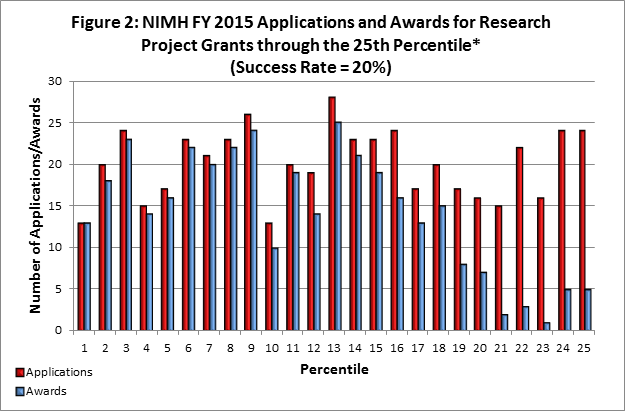
* Almost all applications scoring better than the 10th percentile are funded. Some of the applications through the 10th percentile were not funded due to the funding of previously submitted versions.
- Figure 3 shows the NIMH budget in appropriated (current) versus constant (FY 1998) dollars. Constant dollars are “inflation adjusted” for variations in the purchasing power of the dollar over time. Dollar amounts are adjusted based on the Biomedical Research and Development Price Index (BRDPI). The annual change in BRDPI indicates how much the NIH budget must change to maintain purchasing power similar to FY 1998.
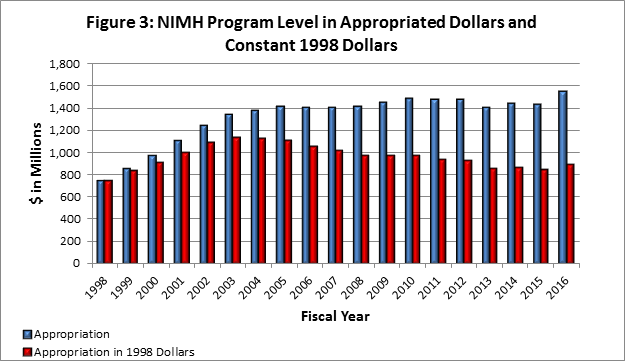
- FY 2016 Budget: On December 18, 2015, President Obama signed an omnibus appropriation (Public Law 114-113) that funds NIH, along with most of the Federal government, through September 30, 2016. The amount provided to NIMH, $1.548 billion, represents an $85.4 million (5.8 percent) increase over the NIMH FY 2015 appropriation. Congress provided increases for all NIH Institutes and Centers (ICs) and additional increases to various ICs for specific programs, such as the BRAIN Initiative , the Precision Medicine Initiative , and for research on Alzheimer’s disease. NIMH’s FY 2016 appropriation includes an increase in funding intended for the President’s BRAIN Initiative.
Prior to the passing of the appropriation, NIH had been operating under a series of short-term Continuing Resolutions (CRs). During these CR periods, NIMH issued non-competing research grant awards at a level below that indicated on the most recent Notices of Award (generally up to 90 percent of the previously committed level). As in previous years, NIMH will adjust these awards to restore them to their committed levels.
NIMH Staff News
- There have been several recent changes to NIMH Leadership.
- Shelli Avenevoli, PhD, is serving as NIMH Acting Deputy Director. Dr. Avenevoli joined NIMH in 2001 and has been heavily involved with a number of key NIMH efforts, among them the revision of NIMH’s Strategic Plan, re-defining the Institute’s approach to supporting research in neurodevelopment and bipolar disorder, and serving as a liaison to other agencies for special initiatives.
- Sarah H. Lisanby, MD, arrived in October and has assumed her role as the Director of the Division of Translational Research (DTR). Dr. Lisanby joined us from Duke University School of Medicine, where she served as Chair of the Department of Psychiatry and Behavioral Sciences, and holds the J.P. Gibbons Professor of Psychiatry endowed chair.
- Sarah Morris, PhD, is serving as the Director of the Research Domain Criteria (RDoC) Unit. Dr. Morris has been active in shaping the RDoC framework while working as a Program Officer for Schizophrenia Spectrum Disorders in DTR.
- Joel Sherrill, PhD, is the new Deputy Director of the Division of Services and Intervention Research. Dr. Sherrill has contributed greatly to NIMH as a Program Official through his efforts at identifying research priorities for federal funding, designing funding initiatives, supporting the implementation of research projects, and nurturing the careers of junior and mid-career scientists.
- Tracy Waldeck, PhD, is serving as the Acting Deputy Director of the Division of Extramural Activities. Dr. Waldeck has accumulated a breadth of knowledge on extramural grants policies and procedures and our use of IT systems to support their management, so as to be a resource not only to NIMH staff and Council members, but to other ICs as well.
- We also say farewell to Kevin Quinn, PhD, Acting Director of the Office of Science Policy, Planning, and Communications, as he will be retiring from NIMH in March. Dr. Quinn has been a valued member of the NIMH team since 1997, when he became the Chief of the Cognitive Neuroscience Program in the Division of Neuroscience and Basic Behavioral Science. In the years following, Kevin assumed a number of key leadership positions, including Chief of the Behavioral Science and Integrative Neuroscience Research Branch and Study Director for the Army Study To Assess Risk and Resilience in Servicemembers (Army STARRS). Dr. Quinn is also a founding member of the RDoC workgroup.
- The NIMH Division of Intramural Research Programs is excited to welcome Mark Histed, PhD, as a new tenure-track investigator. Dr. Histed joined the IRP as Chief of the Unit on Neural Computation and Behavior in January 2016. His laboratory will study cortical circuits and activity patterns linked to behavioral decision making.
Director’s Highlights: NIMH Scientists and Science
Grantee Awards
NIMH is proud to recognize significant achievement and awards received by our current grantees:
- National Academies of Sciences (NAS) Awards in Neuroscience, Psychological and Cognitive Sciences
- Atkinson Prize in Psychological and Cognitive Sciences
- John R. Anderson, PhD (Carnegie Mellon University)
- NAS Award in the Neurosciences
- Mortimer Mishkin, PhD (National Institute of Mental Health)
- Pradel Research Award
- Alex L. Kolodkin, PhD (Johns Hopkins University School of Medicine)
- Atkinson Prize in Psychological and Cognitive Sciences
- Louis S. Goodman & Alfred Gilman Award in Receptor Pharmacology, American Society for Pharmacology and Experimental Therapeutics
- Bryan Roth, MD, PhD (University of North Carolina)
- Award for Lifetime Contribution to the Field of Mental Health, American Public Health Association
- Kimberly Hoagwood, PhD (New York University School of Medicine)
- American College of Neuropsychopharmacology
- Axelrod Mentorship Award
- Robert Post, MD (Bipolar Collaborative Network)
- Efron Research Award
- Joshua Gordon, MD, PhD (Columbia University)
- Eva King Killam Research Award
- Erica Forbes, PhD (University of Pittsburgh)
- Christopher Pittenger, MD, PhD (Yale University)
- Axelrod Mentorship Award
- Diana Forsythe Award, American Medical Informatics Association
- Olga Solomon, PhD (University of Southern California), received the Diana Forsythe Award for her 2014 paper “The social life of health records: Understanding families' experiences of autism,” published in Social Science & Medicine.
Notable NIMH Grants
The following is a selection of the Institute’s most recently funded projects that exemplify our efforts to accelerate mental health research and to advance the NIMH Strategic Plan for Research:
- With nearly 12 million admissions per year, U.S. jails serve as a catchment area for individuals at high risk for suicide. To address this significant problem, Jennifer E. Johnson, PhD (Michigan State University), and Lauren M. Weinstock, PhD (Brown University), have proposed the first randomized evaluation of the Safety Planning Intervention (SPI), a brief, adjunctive, suicide risk reduction intervention developed for suicidal patients presenting to urgent care settings. SPI for at-Risk Individuals in Transition (SPIRIT), a collaboration between NIMH and the National Institute of Justice, will evaluate the effectiveness and cost-effectiveness of SPI for reducing suicide events (attempts, suicide behaviors, suicide-related hospitalizations, and emergency department visits) among 800 detainees in the year following release from jail. Given that roughly ten percent of all suicides in the U.S. occur in the context of a recent criminal legal stressor, reducing suicide risk in the year after jail detention could have a noticeable impact on national suicide rates.
- The representation of external space exists in many elements of our memories, from recalling where you parked your car, to encoding a sequence of locations that help you navigate to work each day. Early stage investigator Lisa Giocomo, PhD (Stanford University), will examine neural circuit organization in the medial entorhinal cortex, a brain region that is highly associated with spatial navigation and memory. Dr. Giocomo proposes to combine in vivo electrophysiology with gene manipulations to delete a set of ion channels (proteins that regulate electrical signaling in a cell) that would then change how grid cells (neurons integral for gauging measures like displacement, distances, and direction) translate the external environment into an internal map of space. These ion channels control something similar to expanding and contracting a map, much as we do on our smartphones with our fingers when we want to zoom in. Her team will test the effects that these ion channel manipulations have on spatial memory and navigation in subsequent behavioral tasks. Understanding how these circuits develop normally and contribute to behavior could lead to new methods for treating the memory-related symptoms and impaired cognitive function of brain disorders.
- Work by David Brent, MD, and Joseph Mann, MD, shows a six-fold increase in risk for suicide attempts in individuals with a family history of major depressive disorder (MDD) with suicide attempts compared to a family history of MDD without suicide attempts. Evidence suggests that risk factors that may contribute to the familial transmission of suicidal behavior include aggression/impulsivity; early-onset depression (beginning around age 10); impairments in memory and executive function; exaggerated cortisol response to stress; and, elevated serotonergic 5-HT1A receptor binding, which can decrease the availability of a neurotransmitter associated with mood. Dr. Brent (University of Pittsburgh) and Dr. Mann (Columbia University) will now test the hypothesis that elevated brain 5-HT1A receptor binding is a biomarker (i.e., a measurable indicator) of suicide risk and lethality that is transmitted in families. Elevated 5-HT1A binding may lower serotonin release, leading to early-onset mood disorders and reactive aggression. This will be the first imaging study to examine a possible biomarker in a population of individuals at high risk for suicide prior to any suicide attempt. If successful, this project will provide a biomarker of suicide risk that will be linked to familial and behavioral indicators of risk.
For more information on these and other grants selected for funding, please visit the NIH RePORTER website .
Recent Findings from NIMH-funded Research
- Suspect Risk Gene for Schizophrenia may Trigger Runaway Synaptic Pruning during Adolescence: Versions of a gene linked to schizophrenia may trigger runaway pruning of the teenage brain’s still-maturing communications infrastructure, NIH-funded researchers recently reported . The team of scientists analyzed the genomes of 65,000 people, looked at 700 postmortem brains, and used a genetically engineered mouse to examine schizophrenia’s strongest known genetic risk factor – the gene C4 (complement component 4) on chromosome 6, as identified by the NIMH-funded Psychiatric Genomics Consortium. Increased expression of a variant of C4 (key in cellular machinery that supports connections between neurons) resulted in a higher risk of an individual developing schizophrenia. The more C4 got switched on, the more synapses got pruned. In humans, pruning occurs as the brain develops to full maturity in the late teens/early adulthood – conspicuously corresponding to the age-of-onset of schizophrenia symptoms. Too much pruning may impair mental function. Future treatments designed to suppress excessive levels of pruning by counteracting runaway C4 in at-risk individuals might prevent a process that could otherwise develop into a psychotic illness.
- Bipolar Schizophrenia Network on Intermediate Phenotypes (BSNIP) Study Supports RDoC Initiative: Three biomarker-based categories, called biotypes, outperformed traditional diagnoses in sorting psychosis cases into distinct subgroups on the basis of brain biology, report researchers from the multi-site BSNIP study. The results lend support to the Institute’s Research Diagnostic Criteria (RDoC) initiative, which frees scientists from designing research based on traditional diagnostic categories, encouraging them to explore groupings based on genomics, behavioral dimensions, physiological traits, or brain imaging findings. These researchers examined key biological and behavioral measures linked to psychosis in 1,872 participants – individuals diagnosed with schizophrenia, schizoaffective disorder, or bipolar disorder with psychosis, their first-degree relatives, and healthy control subjects. Three distinct psychosis-related biotypes emerged that cut across clinical diagnosis boundaries. More precise diagnosis is expected to lead to improved treatments.
- Recovery After an Initial Schizophrenia Episode (RAISE) Outcomes Study Raises Interest in Early Intervention: NIMH-funded researchers have demonstrated that providing comprehensive care for individuals experiencing first episode psychosis (FEP) can improve functional and clinical outcomes, and that these effects are more pronounced with early treatment. The RAISE Early Treatment Program (ETP) is a large-scale comparative effectiveness research study that explored whether using a team-based multicomponent treatment program, referred to as Coordinated Specialty Care (CSC), early in the course of illness would reduce symptoms and prevent the gradual deterioration of functioning that is characteristic of chronic schizophrenia. Over 400 individuals from 34 clinics across 21 states were enrolled in the study. Compared to patients who received usual care, participants in CSC experienced significantly greater improvements in total symptoms, social functioning, work or school involvement, and overall quality of life. Individuals with a shorter duration of untreated psychosis benefited most from CSC treatment, demonstrating that receipt of appropriate care early in the course of FEP is essential to improve outcomes.
New Announcements about Funding Opportunities
NIH electronically posts the NIH GUIDE , a listing of all NIH Funding Opportunity Announcements (FOAs), including requests for applications (RFAs), program announcements (PAs), and important notices for the scientific community. Below is a selection of recently issued FOAs in which NIMH participates. The Funding page on the NIMH website has links to listings of all NIMH FOAs and other resources.
Note: You can subscribe to the NIMH Funding Opportunities ListServ to receive the latest information about RFAs and other research funding opportunities from NIMH, as well as administrative updates and changes to grant policies and procedures. You can also subscribe to a separate listserv to receive weekly e-mails of the NIH GUIDE .
NIMH-Administered Requests for Applications
- BRAIN Initiative: Non-Invasive Neuromodulation - New Tools and Techniques for Spatiotemporal Precision
- Release date: November 24, 2015; Application due date: February 18, 2016
- R01 announcement (RFA-MH-16-810 )
- BRAIN Initiative: Non-Invasive Neuromodulation - Mechanisms and Dose/Response Relationships for Targeted CNS Effects
- Release date: November 24, 2015; Application due date: February 18, 2016
- R01 announcement (RFA-MH-16-815 )
- The Neural Mechanisms of Integrated Emotional and Social Representation
- Release date: January 27, 2016; Application due date: June 3, 2016
- R01 announcement (RFA-MH-17-300 )
- The Neural Mechanisms of Multi-Dimensional Emotional and Social Representation
- Release date: January 27, 2016; Application due date: June 3, 2016
- R21 announcement (RFA-MH-16-815 )
- Exploratory Clinical Trials of Novel Interventions for Mental Disorders
- Release date: March 30, 2015; Application due date: February 17, 2016; June 15, 2016; October 14, 2016
- R33 announcement (RFA-MH-16-400 )
- Exploratory Clinical Trials of Novel Interventions for Mental Disorders
- Release date: May 22, 2015; Application due date: February 17, 2016; June 15, 2016; October 14, 2016
- R61/R33 announcement (RFA-MH-16-406 )
- Pilot Effectiveness Trials for Treatment, Preventive and Services Interventions
- Release date: March 30, 2015; Application due date: February 17, 2016; June 15, 2016; October 14, 2016
- R34 announcement (RFA-MH-16-410 )
- Clinical Trials to Test the Effectiveness of Treatment, Preventive and Services Interventions
- Release date: March 30, 2015; Application due date: February 17, 2016; June 15, 2016; October 14, 2016
- Collaborative R01 announcement (RFA-MH-16-415 )
- R01 announcement (RFA-MH-16-420 )
- Confirmatory Efficacy Clinical Trials of Non-Pharmacological Interventions for Mental Disorders
- Release date: March 30, 2015; Application due date: February 17, 2016; June 15, 2016; October 14, 2016
- R01 announcement (RFA-MH-16-425 )
- NIMH Biobehavioral Research Awards for Innovative New Scientists (NIMH BRAINS)
- Release date: June 11, 2014; Application due date: October 24, 2016
- R01 announcement (RFA-MH-15-600 )
- Applied Research Toward Zero Suicide Healthcare Systems
- Release date: December 11, 2015; Application due date: March 4, 2016; November 2, 2016
- R01 announcement (RFA-MH-16-800 )
NIMH-Collaborative Requests for Applications
- NIH Blueprint for Neuroscience Research Short Courses in Neurotherapeutics Development
- Release date: December 8, 2015; Application due date: February 10, 2016
- R25 announcement (RFA-NS-16-017 )
- BRAIN Initiative: New Technologies and Novel Approaches for Large-Scale Recording and Modulation in the Nervous System
- Release date: November 17, 2015; Application due date: February 24, 2016
- U01 announcement (RFA-NS-16-006 )
- BRAIN Initiative: Optimization of Transformative Technologies for Large Scale Recording and Modulation in the Nervous System
- Release date: November 17, 2015; Application due date: February 24, 2016
- U01 announcement (RFA-NS-16-007 )
- Neuroimaging Informatics Tools and Resources Clearinghouse
- Release date: January 13, 2016; Application due date: March 15, 2016
- U24 announcement (RFA-EB-16-002 )
- BRAIN Initiative: New Concepts and Early - Stage Research for Large - Scale Recording and Modulation in the Nervous System
- Release date: December 11, 2015; Application due date: March 15, 2016
- R21 announcement (RFA-EY-16-001 )
- U.S.-China Program for Biomedical Collaborative Research
- Release date: December 11, 2015; Application due date: March 17, 2016
- R01 announcement (RFA-AI-16-006 )
- NIH Blueprint Training in Computational Neuroscience: From Biology to Model and Back Again
- Release date: January 13, 2016; Application due date: March 18, 2016
- T90/R90 announcement (RFA-DA-16-009 )
- Clinical Sites for the IDeA States Pediatric Clinical Trials Network
- Release date: December 7, 2015; Application due date: April 15, 2016
- UG1 announcement (RFA-OD-16-001 )
- Data Coordinating and Operations Center for the IDeA States Pediatric Clinical Trials Network
- Release date: December 7, 2015; Application due date: April 15, 2016
- U24 announcement (RFA-OD-16-002 )
- Environmental Influences on Child Health Outcomes: Patient Reported Outcomes Research Resource Center Core (ECHO PRO Core)
- Release date: December 7, 2015; Application due date: April 15, 2016
- U24 announcement (RFA-OD-16-003 )
- Environmental Influences on Child Health Outcomes (ECHO) Pediatric Cohorts
- Release date: December 7, 2015; Application due date: April 15, 2016
- UG3/UH3 announcement (RFA-OD-16-004 )
- Environmental Influences on Child Health Outcomes (ECHO) Coordinating Center
- Release date: December 7, 2015; Application due date: April 15, 2016
- U2C announcement (RFA-OD-16-006 )
- BRAIN Initiative: Next-Generation Invasive Devices for Recording and Modulation in the Human Central Nervous System
- Release date: September 17, 2015; Application due date: April 26, 2016
- UG3/UH3 announcement (RFA-NS-16-009 )
- BRAIN Initiative: Clinical Studies to Advance Next-Generation Invasive Devices for Recording and Modulation in the Human Central Nervous System
- Release date: September 17, 2015; Application due date: April 26, 2016
- UH3 announcement (RFA-NS-16-010 )
- Revisions to Add Biomedical Big Data Training to Active Institutional Training Grants
- Release date: April 22, 2014; Application due date: July 28, 2016
- T32 announcement (RFA-HG-14-005 )
Future Research Directions
Concept Clearances for Potential New Research Initiatives
This listing of potential future initiatives is meant to provide the earliest possible alert to the field of our research interests and of potential upcoming announcements to solicit that research. While NIMH plans to proceed with these initiatives, their publication and timing are not certain and depend on sufficient funding. The titles and brief descriptions are consistent with the information available at the time of concept clearance. The resultant FOAs may differ from the concepts in the final wording of their titles or other aspects. To send questions about a specific concept, follow the “Submit Comments” link at the bottom of the description.
- Applied Research towards Zero Suicide Healthcare Systems
- Role of Myeloid Cells in Persistence and Eradication of HIV-1 Reservoirs from the Brain
For more information, please see recent NAMHC-approved concepts, recent public venue-approved concepts, and past NAMHC meetings, which also contains links to meeting agendas, minutes, and Inside NIMH (Director’s Reports).
NIMH-Sponsored Meetings
- NIMH Alliance for Research Progress: NIMH convened the 22nd meeting of the NIMH Alliance for Research Progress on September 16, 2015, in Rockville, MD. Alliance participants represent wide-ranging perspectives, including those of consumers, providers of mental health services, and family members. The annual meeting is a reflection of NIMH’s commitment to communicating often with its constituents and, as importantly, to soliciting feedback from them, including their views of public health needs and their overall assessment of how NIMH plans, priorities, and activities are addressing those needs. Then-NIMH Director Dr. Tom Insel updated Alliance members on recent research advances and activities, including the RAISE Early Treatment Program and the Precision Medicine Initiative , and held discussions on depression, resiliency, and the barriers to receiving mental health treatments.
- Reducing the Incidence of Suicide in Indigenous Groups: Strengths United through Networks (RISING SUN): The NIMH Office for Research on Disparities and Global Mental Health launched the RISING SUN initiative with a workshop in Anchorage, AK, on September 19-20, 2015. The RISING SUN initiative aims to engage a diverse set of stakeholders to reduce the incidence of suicide among indigenous communities by creating a toolkit of measures to evaluate correlates and outcomes of suicide prevention interventions across the Arctic. The workshop was co-sponsored by the U.S. Department of State, the Norwegian Public Health Institute, the Canadian Institutes of Health Research, the Institute of Public Health-Denmark, the Inuit Circumpolar Council, and the Arctic Research Commission. RISING SUN is a project under the U.S. chairmanship of the Arctic Council, the intergovernmental forum that promotes cooperation among Arctic States, Arctic Indigenous communities, and other Arctic residents.
- Brain Stimulation Based Neural Circuit Modeling: Linking levels of Analysis: This satellite symposium at the 2015 Society for Neuroscience Meeting in Chicago, IL, brought together basic neuroscientists, computational modelers, and clinical investigators to discuss how to connect large scale computational modeling with translational neuroscience. The overall goal was to increase understanding of how to improve basic network organization using non-invasive brain stimulation and enhance cognitive capabilities in clinical disorders. Increasing our mechanistic understanding of how weak global changes interact with large-scale neural dynamics will also facilitate the design of more effective and safer neuromodulatory devices for the improvement of brain function in health and disease.
Update on Electronic Research Administration (eRA) Activities
- NIH has announced changes to policies, instructions, and forms for grant applications starting in 2016. Detailed instructions can be found in Notice NOT-OD-16-004 , and are described in the posted guide and FAQs . Specifically, these changes are related to: vertebrate animals (NOT-OD-16-006 ); research training (NOT-OD-16-007 ); font requirements (NOT-OD-16-009 ); definition of a child (NOT-OD-16-010 ); rigor and transparency in research (NOT-OD-16-011 and NOT-OD-16-012 ); data safety monitoring; appendices; and biosketch clarifications (NOT-OD-16-004 ).
- The NIH Office of Policy for Extramural Research Administration (OPERA) announced the release of the revised NIH Grants Policy Statement (Oct/Nov 2015). This revision supersedes, in its entirety, the previous NIH Grants Policy Statement from March, 2015, as a standard term and condition of award; a summary of significant changes is now available.
- NIH announced changes to post-award forms and instructions (NOT-OD-16-005 ). These changes also apply to the revised Ruth L. Kirschstein National Research Service Award (NRSA) forms .
- Fellowship applications may now be submitted via Application Submission System & Interface for Submission Tracking (ASSIST; NOT-OD-16-023 ). Although the use of ASSIST is optional for these mechanisms, the website allows for collaboration of multiple users and pre-submission validation of many NIH and Grants.gov business rules.
- The Commons Help Desk has been rebranded as the eRA Service Desk ; Commons users may notice this change on screens, email notifications, web pages, and other resources. The new name reflects the expansion to support ASSIST and other eRA services. The eRA Service Desk will continue to provide high-quality support for eRA Commons and its modules. A new eRA Commons User Guide was released in October, and specific changes are described in the eRA Commons Release Notes .
For more information on all of these updates, please see the NIH eRA News and Events page .
Questions? Contact the eRA Service desk . Note that contacting this help desk is the only way to document problems with an electronic grant application submission. Evidence of this contact is the only way to be eligible for any special consideration by the Center for Scientific Review (CSR) Division of Receipt and Referral, should you run into a system problem with Grants.gov or with eRA that is beyond your control.
Research Training and Career Development
The latest news about Research Training and Career Development at the NIMH and the NIH:
- Change in NIH Policy regarding Salary Allowance on K08 and K23 Awards: In 2014, a Working Group of the Advisory Committee to the NIH Director issued a report on the Physician Scientist Workforce that made recommendations regarding funding and training of physician scientists in order to attract and retain well-qualified individuals in research careers. On December 17, 2015, NIH announced a new policy on salary and research cost allowances for K08 and K23 awards (NOT-OD-16-032 ) effective with new (Type 1) applications due on and after February 12, 2016. With this policy change, NIMH will contribute up to $100,000 toward the K08 or K23 awardee’s salary to offset the requested level of effort that will be devoted to research and career development. Please note that this policy applies to new (Type 1) application and to all non-competing continuation (Type 5) applications submitted for FY 2017 funding.
- Formal Instruction in Rigorous Experimental Design: NIH recently announced that formal instruction in rigorous experimental design and transparency to enhance reproducibility will be required as part of institutional training grants (e.g., T32) and individual fellowships (e.g., F30, F31, F32) as early as FY 2017 (NOT-OD-16-034 ). Training program directors and mentors are encouraged to begin to develop substantive instructional plans now so that they are in place in advance of the implementation deadline. For more information see the NIH portal on data rigor .
We are interested in feedback from the community; comments or suggestions related to NIMH’s support for research training and career development may be directed to NIMH_Training@mail.nih.gov. You may also contact NIMH Program Staff with questions or comments.
NIMH Science Updates
The latest news and updates from NIMH-supported research:
- Genome-Wide Study Yields Markers of Lithium Response (January 28, 2016)
- Experimental Combination Surprises with Anti-HIV Effectiveness (January 20, 2016)
- Circuit Tweak Boosts Social Memory in Mice (January 7, 2016)
- Biomarkers Outperform Symptoms in Parsing Psychosis Subgroups (December 8, 2015)
- Biomarker for Brain Excitability May Help Track Medication Effect (November 20, 2015)
- Combating Early Death in People with Serious Mental Illness (November 4, 2015)
- Federal Agencies Partner to Promote Coordinated Services for Patients with First Episode Psychosis (October 29, 2015)
- Blog, Video Spotlight NIMH Neuroscience Trainee (October 15, 2015)
- Webinar: Facts and Myths about RDoC (September 29, 2015)
- National Suicide Prevention Month: Update 2015 (September 28, 2015)
- RDoC Joins the Twitterverse (September 23, 2015)
- Embracing the SPIRIT of Reducing Suicide (September 21, 2015)
Publicizing NIMH research is a communal responsibility. Please help us spread the word about the results of NIMH funding by acknowledging our support of your research, for example, in journal articles (citing your NIMH award by number when possible) and other communications. NIMH has two primary methods of getting the word out: press releases and science updates. All releases and updates are posted to the Science News section of the NIMH Web site. These are also distributed to the public through a mailing list .
Connect with NIMH
Inside NIMH is produced by the National Institute of Mental Health. For more information about the Institute, visit our website at https://www.nimh.nih.gov. For comments and suggestions about Inside NIMH, please contact the NIMH Webmaster. The material in this newsletter is not copyrighted, and we encourage its use or reprinting.
Our newest effort to reach our stakeholders is a service that allows you to subscribe for updates sent directly to your email inbox on the NIMH topics of your choice. In addition to our email newsletters and RSS updates, please also visit NIMH on Twitter , Facebook , and YouTube , where we highlight Science Updates, Press Releases, and other timely matters.
