2013 Autumn Inside NIMH

Table of Contents
- Message from the NIMH Director
- Director’s Highlights: NIMH Science and Scientists
- New Announcements about Funding Opportunities
- Future Research Directions
- Update on Electronic Research Administration (eRA) Activities
- Research Training and Career Development
- Director’s Blog
- NIMH Science Updates
- Connect with NIMH
Welcome to the latest edition of Inside NIMH. We publish Inside NIMH in conjunction with each meeting of the National Advisory Mental Health Council, which advises the Secretary of Health and Human Services, the Director of the National Institutes of Health, and the Director of NIMH on all policies and activities relating to the conduct and support of mental health research, research training, and other programs of the Institute. In addition, check out the Director’s blog on our website for regular updates on timely topics at NIMH. I hope you find this information interesting and helpful. Please let us know if you have questions or comments on this edition.
Sincerely,
Thomas R. Insel, M.D.
Director, National Institute of Mental Health
If you wish to unsubscribe, subscribe, or change your email address, please visit the Inside NIMH subscription page or contact the NIMH Webmaster.
I. Message from the NIMH Director
In the Spring 2013 issue of Inside NIMH, I described several new initiatives including efforts for early psychosis, the NIH Brain Research for Advancing Innovative Neurotechnologies (BRAIN) Initiative, and new priorities for NIMH clinical trials. In this issue, I will briefly update each of these areas and note three other areas that NIMH will be prioritizing.
Follow-ups from Spring 2013
- Early Psychosis Prediction and Prevention (EP3): For more than a decade, NIMH-funded research has focused on the prodrome, the high-risk period preceding the onset of the first psychotic episode of schizophrenia. Based on the success of a multi-site cooperative agreement, the North American Prodrome Longitudinal Study (NAPLS), and many related studies, we will be focusing on early prediction and prevention of psychosis, which we are calling “EP3,” as a priority issue for the Institute in the next five years. Approximately 100,000 adolescents and young adults have a first psychotic episode each year in the US. EP3 will develop biomarkers for prediction and new interventions for preemption to reduce this number significantly. The first two funding announcements for EP3 have already been released: Research to Improve the Care of Persons at Clinical High Risk for Psychotic Disorders (RFA-MH-14-210 , RFA-MH-14-211 , and RFA-MH-14-212 ) and Reducing the Duration of Untreated Psychosis in the United States (PAR-13-187 and PAR-13-188 ).
- The BRAIN Initiative: In April 2013, the President announced the BRAIN Initiative , declaring neuroscience research as the “next great American project.” The BRAIN Initiative, which includes $40 million in funding from NIH in fiscal year (FY) 2014, will support the development of new technologies for understanding how neural circuits link to behavior. Leveraging recent progress in molecular biology and imaging, we can now make rapid progress on understanding neural circuits, including the circuits involved in mental illness. Based on four recent public meetings on the BRAIN Initiative, a workgroup of the Advisory Council to the Director of NIH has just issued an interim report of recommendations. NIMH will contribute $7.5 million to the BRAIN initiative in FY 2014.
- New Clinical Trials: A review by a Council Workgroup (From Discovery to Cure), the shift away from treatment development within industry, and the increasing morbidity and mortality from mental illness (US Burden of Disease Collaborators, 2013 ) collectively suggest a reappraisal of the NIMH clinical trial portfolio. Last year, NIMH supported numerous clinical trials; however, an insufficient number of these trials are validating new targets or mechanisms of disease, and many are hindered by heterogeneous diagnostic categories. Since May 2013, NIMH has launched new contracts for Fast-Fail Trials (FAST) in Autism Spectrum Disorders (FAST-AS), Mood and Anxiety Spectrum Disorders (FAST-MAS), and Psychotic Spectrum Disorders (FAST-PS), as well as Rapidly-Acting Treatments for Treatment-Resistant Depression (RAPID) focused on rapid treatments of depression. We are preparing new funding announcements related to clinical trials with a focus on experimental therapeutics (NOT-MH-13-022 ). Please note that NIMH is terminating its support of clinical trials using the collaborative R34 mechanism (NOT-MH-13-023 ) and will not be supporting clinical trials under certain broad NIH parent announcements.
New Priorities
- Post-traumatic Stress Disorder (PTSD) and Suicide in the Military: In a speech to wounded veterans in Orlando on August 10, 2013, President Obama announced the National Research Action Plan (NRAP), a coordinated effort by the Departments of Defense (DOD), Veterans Affairs, Health and Human Services (HHS), and Education in response to last year’s Executive Order that called for improved access to mental health services for veterans, service members, and military families. NRAP provides a comprehensive approach to accelerating research on traumatic brain injury and PTSD, as well as strategies for preventing suicide among veterans and active duty personnel. The Departments have jointly reviewed the state of the science, the current research portfolios, and the opportunities for progress. Together, we have committed to transforming the research landscape to accelerate progress. Short-term objectives include the development of common data elements to facilitate data sharing and a focus on biomarkers to predict PTSD. NRAP also called for turning the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) project, a collaborative NIMH-DOD project now entering its fifth and final year of funding, into a longitudinal study along the lines of the Framingham study of cardiovascular risk.
- Suicide Prevention: Last Fall, the National Action Alliance for Suicide Prevention released the revised National Strategy for Suicide Prevention . Recognizing that the number of suicides in this country continues to climb (in contrast to the rate of homicides and traffic fatalities), this report called for several objectives, including a prioritized research agenda. Based on an intensive process of gathering input from many stakeholders and experts, a suicide prevention plan is being developed with a goal of shaping research to more efficiently reduce morbidity (attempts) and mortality (deaths). Along with NRAP, this new agenda will provide a strategic plan for suicide research. A first request for applications (RFA) has been released: Pediatric Suicide Prevention in Emergency Departments (RFA-MH-14-070 ).
- Replication: Following recent reports that much of the preclinical research supported in academia cannot be replicated by scientists in industry, NIH has been looking carefully at ways to enhance the reproducibility and transparency of research findings. The National Institute of Neurological Disorders and Stroke (NINDS) and the National Cancer Institute (NCI) held meetings on this topic, and NIH convened an internal workgroup to explore barriers and opportunities to improving the rigor of science we support. As a first step, NIH will develop some new training tools to focus on experimental bias and research design. A number of pilot projects are underway as well, including a process to assess the scientific premise of Phase I clinical trials, a focus on developing a checklist for scientific rigor, and re-engineering the biosketch to reduce the pressure for multiple publications. While much of the attention regarding lack of replication has concerned neurologic research and cancer, NIMH has an equal interest in ensuring the reproducibility and transparency of its research and will be discussing this issue with Council.
Budget Overview
- FY 2013 Budget: We are currently on track to award 502 new and competing research project grants (RPGs) in FY 2013. This number is lower than the 529 that we had planned to fund, mostly due to a reduction in the number of grants from the Division of AIDS Research. Within the non-AIDS portion of the NIMH portfolio, the number of grants in FY 2013 is expected to be 448, slightly more than our projection of 444, but considerably less than the 517 awarded in FY 2012. The overall success rate for competing RPGs in FY 2013 is currently 18%. The projected success rate for Early Stage Investigators (ESIs) is 21% compared to 25% in FY 2012. We anticipate funding a total of 87 New Principal Investigators (PIs) in FY 2013 compared to 115 in FY 2012. One reason we funded fewer ESIs and New PIs this year compared to last year is that we were able to fund a considerable number of RPGs planned for FY 2013, including 5 ESIs and 11 New PIs, with-end-of year funds in FY 2012. An impact of FY 2013 sequestration is that we will not be funding FY 2014 grants early at the end of this fiscal year.
- FY 2014 Budget: FY 2014 will almost certainly begin under a continuing resolution (CR), at a level that is 2.4% below FY 2013. As in the past, while operating under a CR, non-competing grants will be awarded at levels below committed amounts, likely at 90%. NIMH’s policy regarding future year commitments (FY 2014 and beyond) will be determined after we receive a full year appropriation for FY 2014.
NIMH Staff News
We would like to welcome some recent additions to the NIMH extramural staff:
- Nitin Gogtay, MD, of NIMH’s Division of Intramural Research Programs (IRP), has joined the NIMH Office of the Director as Associate Director for Clinical Research. For the past decade Dr. Gogtay has been a staff psychiatrist in the Child Psychiatry Branch, playing an instrumental role in the Childhood Onset Schizophrenia Study, and the effort to map normal healthy brain development from an early age to adulthood.
- Charlene E. Le Fauve, PhD, has joined NIMH as the Deputy Director for the Office for Research on Disparities and Global Mental Health (ORDGMH). Dr. Le Fauve’s Federal experience spans nearly 15 years, with her most recent position at HHS in the Immediate Office of the Secretary, where she worked as a Senior Policy Coordinator and Lead for the Centers for Medicare and Medicaid Services in the office of the Executive Secretariat.
Return to Top
II. Director’s Highlights: NIMH Science and Scientists
Grantee Awards
- The Albert Lasker Basic Medical Research Award has been awarded to Thomas C. Südhof, MD, and Richard H. Scheller, PhD, for discoveries concerning the molecular machinery and regulatory mechanism that underlie the rapid release of neurotransmitters. Both of these investigators have a long history of research support and service with NIMH.
- Thomas C. Südhof, MD, is the Avram Goldstein Professor of Molecular and Cellular Physiology and Howard Hughes Medical Institute Investigator (HHMI) in the Departments of Neurology, Psychiatry, and Behavioral Sciences at Stanford University. Dr. Südhof is a current NIMH grantee and has served on several study sections at the NIH Center for Scientific Review, in addition to the Molecular, Cellular, and Developmental Neuroscience study section at NIMH. Dr. Südhof’s honors include an NIMH MERIT Award, the W. Alden Spencer Award, the Kavli Prize, and membership in the National Academy of Sciences.
- Richard H. Scheller, PhD, is the Executive Vice President of Research and Early Development at Genentech in San Francisco, CA. During his academic research career, Dr. Scheller received research support from NIMH, served on the NIMH Molecular, Cellular, and Developmental Neuroscience study section, on the National Advisory Mental Health Council from 1996-1999, and was an HHMI investigator from 1990-2001. Dr. Scheller’s honors include an NIMH MERIT Award, the W. Alden Spencer Award, the Kavli Prize, and membership in the National Academy of Sciences.
New NIMH Science Advances
Recent findings from NIMH-funded research that are helping to advance the field:

Genetic Overlap between Mental Disorders: Performed by the Cross Disorders Group of the Psychiatric Genomics Consortium (PGC), the largest genome-wide study of its kind has determined how much five major mental illnesses are traceable to the same common inherited genetic variations. In this multi-site study, researchers found that the overlap was highest between schizophrenia and bipolar disorder; moderate for bipolar disorder and depression and for ADHD and depression; and low between schizophrenia and autism. Overall, common genetic variation accounted for 17-28% of risk for the illnesses. These findings of shared genetic risk among traditional psychiatric diagnoses are of relevance to NIMH’s Research Domain Criteria (RDoC) project, which is developing a classification system for mental health research based more on underlying biological mechanisms than on symptoms.
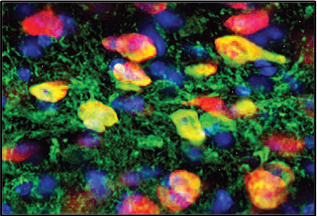
Source: Feng Zhang, PhD, Broad and
McGovern Institutes, MITNew Technology can Rapidly Start or Stop Gene Expression: With funding from the NIH Transformative Research Award Program and NIMH, Feng Zhang, PhD , and his colleagues developed a new technology that can rapidly start or stop the expression of a gene of interest. This new technique, based on optogenetics which uses proteins that change their function in response to light, allows researchers to precisely control the timing and duration of gene expression. In addition, Dr. Zhang and his colleagues used this light-sensitive technique to alter the degree to which other genetic material in the cells could modify regular gene expression, for example epigenetic modifications. This technology will make it easier to determine the roles of specific genes, in particular those involved in learning and memory. This work provides another example of the types of research that NIMH hopes to cultivate through the BRAIN Initiative .
Notable NIMH Grants
Here is a selection of the Institute’s most recently funded projects that exemplify our efforts to accelerate mental health research and to advance the NIMH Strategic Plan:
- Recent research has identified the occurrence of mutations in a protein complex, neuron-specific Brg1-associated factor (nBAF), in several disorders associated with intellectual disability, including sporadic cases of autism with no family history of autism or related disorders. In a series of studies, Marcelo Wood, PhD , (University of California, Irvine) is examining the role of a subunit of this complex, BAF53b, in long-term memory and connection strength between brain cells, as well as the impact of BAF53b on genes associated with memory consolidation. These studies aim to clarify how the nBAF complex affects memory processes, and may improve our understanding of how mutations in this complex lead to cognitive impairments associated with intellectual disorders in humans.
- Psychosis typically has an onset in early adulthood, but some individuals experience psychosis in adolescence, a developmental period that is characterized by significant change in brain structure and function. BRAINS Award (Biobehavioral Research Awards for Innovative New Scientists ) recipient Katherine Karlsgodt, PhD , (Feinstein Institute for Medical Research) is investigating brain circuits involved in reward, working memory, and decision-making in typically developing adolescents and those with a clinical diagnosis of psychosis. This longitudinal study may provide crucial information about neural systems for decision-making in typical and pathological development, and Dr. Karlsgodt aims to identify biomarkers that predict trajectories of psychosis and sensitivity to treatment.
- Robert Miller, MD , (University of Minnesota) is proposing a new way of evaluating patients with schizophrenia based on a simple, non-invasive ophthalmological recording technique known as the electroretinogram (ERG). Abnormal regulation of N-methyl-D-Aspartate (NMDA) receptors has been implicated as one cause of schizophrenia, and Dr. Miller and his colleagues propose using a variant of the conventional ERG, called the pattern ERG (pERG), that is designed to reveal NMDA receptor activity of retinal cells. Indeed, preliminary data suggest that the pERG is characteristically different in individuals with schizophrenia compared to controls. These studies may reveal new insights into the origins of schizophrenia, and may provide a broadly useful experimental and clinical tool for diagnosis.
- Although millions of health and mental health “apps” have been downloaded, there is relatively little information regarding user access, engagement, and impact on mood, cognition, and daily functioning. Patricia Arean, PhD , and Adam Gazzaley, MD, PhD , (University of California, San Francisco) are using novel approaches, including social networking, search engines, and download-based methods (app stores), to identify and recruit individuals with depression for a study examining the feasibility of two distinct mental health apps: one utilizing evidence-based psychotherapy (Problem Solving Therapy), and one utilizing a cognitive training approach. This study is being conducted entirely over smartphone and tablet devices, and will advance research to determine if mental health apps are used as intended, and if they really help with mood and thinking.
For more information on these and other grants selected for funding, please visit the NIH RePORTER website.
Return to Top
III. New Announcements about Funding Opportunities
Each week, NIH electronically distributes the NIH GUIDE , a listing of all NIH Funding Opportunity Announcements (FOAs) that include requests for applications (RFAs), program announcements (PAs), and important notices for the scientific community. Below is a selection of recently issued FOAs in which NIMH participates. The Funding page on the NIMH website has links to listings of all NIMH FOAs and other resources.
Note: You can subscribe to the NIMH Funding Opportunities ListServ to receive the latest information about RFAs and other research funding opportunities from NIMH, as well as administrative updates and changes to grant policies and procedures. You can also subscribe to a separate listserv to receive weekly emails of the NIH GUIDE .
NIMH-Administered Requests for Applications
- Gut-Microbiome-Brain Interactions and Mental Health
- Release date: March 8, 2013; Expiration date: October 10, 2013
- R21/R33 announcement (RFA-MH-14-080 )
- Pediatric Suicide Prevention in Emergency Departments
- Release date: April 15, 2013; Expiration date: October 18, 2013
- U01 announcement (RFA-MH-14-070 )
- Services Research for Autism Spectrum Disorder across the Lifespan (ServASD): Research on Early Identification and Linkage to Services for ASD
- Release date: May 30, 2013; Expiration date: October 22, 2013
- R01 announcement (RFA-MH-14-100 )
- Services Research for Autism Spectrum Disorder across the Lifespan (ServASD): Pilot Research on Services for Transition-Age Youth
- Release date: May 30, 2013; Expiration date: October 22, 2013
- R34 announcement (RFA-MH-14-101 )
- Services Research for Autism Spectrum Disorders across the Lifespan (ServASD): Pilot Studies of Services Strategies for Adults with ASD
- Release date: May 30, 2013; Expiration date: October 22, 2013
- R34 announcement (RFA-MH-14-102 )
- Mentored Career Development Award to Build Research Capacity in Global Mental Health
- Release date: June 18, 2013; Expiration date: October 22, 2013
- K01 announcement (RFA-MH-14-120 )
- Biobehavioral Research Awards for Innovative New Scientists (BRAINS)
- Release date: June 18, 2012; Expiration date: October 23, 2013
- R01 announcement (RFA-MH-13-110 )
- Improving Health and Reducing Premature Mortality in People with Severe Mental Illness
- Release date: March 12, 2013; Expiration date: November 7, 2013
- R01 announcement (RFA-MH-14-060 )
- Research to Improve the Care of Persons at Clinical High Risk for Psychotic Disorders
- Release date: August 7, 2013; Expiration date: January 6, 2014
- Collaborative R01 announcement (RFA-MH-14-210 )
- R01 announcement (RFA-MH-14-211 )
- R34 announcement (RFA-MH-14-212 )
- National Cooperative Reprogrammed Cell Research Groups (NCRCRG) to Study Mental Illness
- Release date: May 8, 2013; Expiration date: January 8, 2016
- U19 announcement (PAR-13-225 )
- Reducing the Duration of Untreated Psychosis in the United States
- Release date: April 10, 2013; Expiration date: May 8, 2016
- R01 announcement (PAR-13-187 )
- R34 announcement (PAR-13-188 )
NIMH-Collaborative Requests for Applications
- NIH Transformative Research Awards
- Release date: July 26, 2013; Expiration date: October 4, 2013
- R01 announcement (RFA-RM-13-008 )
- NIH Pioneer Award Program
- Release date: August 8, 2013; Expiration date: October 18, 2013 (Future dates: October 10, 2014 and October 9, 2015)
- DP1 announcement (RFA-RM-13-006 )
- NIH Director's New Innovator Award Program
- Release date: August 8, 2013; Expiration date: October 25, 2013 (Future dates: October 17, 2014 and October 16, 2015)
- DP2 announcement (RFA-RM-13-007 )
- Research on the Role of Epigenetics in Social, Behavioral, Environmental and Biological Relationships, throughout the Life-Span and across Generations
- Release date: May 17, 2013; Expiration date: November 13, 2013
- R21 announcement (RFA-TW-13-002 )
- Short Courses on Innovative Methodologies in the Behavioral and Social Sciences
- Release date: May 3, 2013; Expiration date: November 14, 2013
- R25 announcement (RFA-OD-13-009 )
- DNA Sequencing Core for an Undiagnosed Diseases Network (UDN)
- Release date: August 7, 2013; Expiration date: November 19, 2013
- U01 announcement (RFA-RM-13-018 )
- Centers of Excellence for Big Data Computing in the Biomedical Sciences
- Release date: July 22, 2013; Expiration date: November 20, 2013
- U54 announcement (RFA-HG-13-009 )
- NIH Health Care Systems Research Collaboratory - Demonstration Projects for Pragmatic Clinical Trials Focusing on Multiple Chronic Conditions
- Release date: August 7, 2013; Expiration date: December 2, 2013
- UH2/UH3 announcement (RFA-RM-13-012 )
- Short-term Mentored Career Enhancement Awards in the Basic Behavioral and Social Sciences: Cross-Training at the Intersection of Animal Models and Human Investigation
- Release date: March 1, 2013; Expiration date: December 11, 2013
- K18 announcement (RFA-DA-14-002 )
- Library of Integrated Network-Based Cellular Signatures (LINCS): Perturbation-Induced Data and Signature Generation Centers
- Release date: August 19, 2013; Expiration date: December 19, 2013
- U54 announcement (RFA-RM-13-013 )
- NIH Administrative Supplements to Recover Losses Due to Hurricane Sandy Under the Disaster Relief Appropriations Act - Non-Construction
- Release date: April 15, 2013; Expiration date: January 14, 2014
- Administrative Supplement announcement (RFA-OD-13-199 )
- NIH Director's Early Independence Awards
- Release date: August 14, 2013; Expiration date: January 31, 2014
- DP5 announcement (RFA-RM-13-009 )
- Development of Highly Innovative Tools and Technology for Analysis of Single Cells
- Release date: March 13, 2013; Expiration date: January 8, 2016
- R43/R44 announcement (PA-13-140 )
Return to Top
IV. Future Research Directions
Concept Clearances for Potential New Research Initiatives
This listing of potential future initiatives is meant to provide the earliest possible alert to the field of our research interests and of potential upcoming announcements to solicit that research. While NIMH plans to proceed with these initiatives, their publication and timing are not certain and depend on sufficient funding. The titles and brief descriptions are consistent with the information available at the time of concept clearance. The resultant FOAs may differ from the concepts in the final wording of their titles or other aspects. To send questions about a specific concept, follow the “Submit Comments” link at the bottom of the description.
- First-Generation RDoC Standard Data Elements
- Development of a BrainSpan RNA-seq Browser
- Toward Early Prediction of Psychosis: Collaborative Research on Developmental Risk in 22q11.2 Deletion Syndrome
- Secondary Data Analyses to Explore RDoC Domains
- Post-Traumatic Stress Disorder: Heterogeneity and Translation
- Imaging Data and Biomarkers
For more information, please see recent NAMHC-approved concepts, recent public venue-approved concepts, and past NAMHC meetings, which also contains links to meeting agendas, minutes, and Director’s Reports.
NIMH-sponsored Meetings
- NIMH Alliance for Research Progress: The NIMH Office of Constituency Relations and Public Liaison convened the nineteenth meeting of the Alliance on July 12, 2013 in Bethesda, MD. Leaders from national organizations focused on mental illness heard presentations about the state of NIMH from Dr. Insel, a behavioral weight loss intervention for persons with serious mental illness, patient empowerment efforts in other disease areas, the use of brain imaging as a potential biomarker for more personalized interventions in mental illness, and prospects for novel diagnostics and therapeutics through better understanding of brain circuitry.
- Advancing Knowledge on Mental Health Disparities: The NIMH Office for Research on Disparities and Global Mental Health hosted two webinars on Advancing Knowledge on Mental Health Disparities on August 13 and 27, 2013. Presentations included:
- The Role of Social Networks in Treatment Engagement for Minorities
- Advancing Knowledge on Mental Health Disparities for Perinatal Depression Through a Network-based Approach
- Randomized Trial Achieving Healthy Lifestyles in Psychiatric Rehabilitation
- Understanding Mechanisms of Mental Health Care Disparities
- Supplement to the Center for Culture, Trauma, and Mental Health Disparities.
- Pediatric Pharmacogenomics Symposium: On September 11, 2013, the NIMH Division of Developmental Translational Research and the NIMH Office of Genomics Research Coordination co-hosted a symposium on the state of pediatric pharmacogenomics - the science of using information about children’s genetic sequences to predict their responses and responsiveness to treatment. Presentations by leaders in the field covered recent advances, current hurdles, ethical and regulatory considerations, how to use pharmacogenomics results in clinical practice, and how to change research practice.
Return to Top
V. Update on Electronic Research Administration (eRA) Activities
Electronic Grant Application Submission News
- Personal Profile in the eRA Commons
- There is a new interface for the Personal Profile module in eRA Commons. For assistance in keeping your personal data in this profile up-to-date, please see the Personal Profile Overview demonstration video (July 25, 2013) and visit the Commons Personal Profile Online Help System .
- Change of Institution
- A Change of Institution (Type 7) application is a non-competing process that allows an active grant to be moved from one institution to another. To learn more about this process, please see the new Change of Institution Online Help System and eRA Commons Frequently Asked Questions about Change of Institution/Relinquishing Statement (Type 7s) .
- Research Performance Progress Report:
- Investigators are now required to use the Research Performance Progress Report (RPPR) module for all awards issued under the Streamlined Noncompeting Award Process (SNAP) and all Fellowship progress reports (for F grants with budget starts after July 1, 2013). Please see the official Guide Notice NOT-OD-13-035 for more information.
- RPPR will also eventually replace the use of the PHS 2590 for non-SNAP awards. For more information, please see the RPPR web page and the Guide Notice NOT-OD-13-061 .
- Small Business Innovation Research / Small Business Technology Transfer Reauthorization Act
- There are some additional requirements for Small Business Innovation Research / Small Business Technology Transfer (SBIR/STTR ) applications due to the SBIR/STTR Reauthorization Act. Under this Act, all small business applicants are required to register with the Company Registry Database at the time of application. Applicants will be given a warning if their registration file is not attached to a SBIR/STTR application.
- Internet Assist Review System
- The Internet Assist Review (IAR) system has been upgraded, and now includes a new online help system, the IAR Online Help System for Reviewers . In the next release, the online help system will be integrated within the application screens. Please note that the IAR for Reviewers User Guide (PDF) is still available.
For more information on all of these updates, please see the NIH eRA News and Events page .
Questions? Contact the eRA help desk . Note that contacting this help desk is the only way to document problems with an electronic grant application submission. Evidence of this contact is the only way to be eligible for any special consideration by the CSR Division of Receipt and Referral, should you run into a system problem with Grants.gov or with eRA that is beyond your control.
Return to Top
VI. Research Training and Career Development
Individual Development Plans and Broadening Scientific Training
The 2012 report from the Biomedical Workforce Task Force of the NIH Advisory Committee to the Director (ACD) included recommendations for actions that the NIH should take to support a sustainable biomedical infrastructure and workforce. The last issue of Inside NIMH highlighted the upcoming change in duration of the application eligibility window (from 5 to 4 years) for the NIH Pathway to Independence (K99/R00) Award (NOT-OD-13-050 ). Here are updates on two other components of the proposed implementation plan :
- Individual Development Plans: The Biomedical Workforce Task Force acknowledged that biomedical graduate students and postdoctoral researchers need better preparation in order to participate successfully in the evolving US economy. The NIH is now encouraging institutions to implement individual development plans (IDPs) for all NIH-supported graduate students and postdoctoral researchers, as noted in a recently issued NIH Guide Notice (NOT-OD-13-093 ). IDPs are viewed as a tool to assist individuals in achieving their career goals as members of the biomedical research workforce.
- Broadening Scientific Training: Recognizing that PhD graduates can contribute to the biomedical research enterprise through a variety of career paths, the NIH launched the Strengthening the Biomedical Research Workforce program through the Common Fund . This program will provide support for academic institutions to develop innovative approaches that complement traditional biomedical research training. The first awards in this program will be issued this fall, with additional awards anticipated in 2014. The NIH Biomedical Research Workforce web page has information about efforts to implement the recommendations of the Task Force. NIMH encourages Institutional Training (T32) Program Directors to consider including career planning discussions and development of transferable professional skills – such as effective oral and written communication skills with a broad range of potential audiences, project management skills, “people” skills, as well as grant-writing skills – in their programs.
Please contact NIMH Program Staff with questions or comments.
We’re interested in feedback from the community; comments or suggestions related to NIMH’s support for research training and career development may be directed to NIMH_Training@mail.nih.gov.
Return to Top
VII. Director’s Blog
The following blogs by former NIMH Director Thomas Insel are no longer available.
- In Vitro Veritas? (September 16, 2013)
- With more than 100 common gene variants recently implicated in schizophrenia and autism, the problem now is to pinpoint how they might change brain circuits.
- Accessing and Assessing Science: From PLOS to DORA (September 5, 2013)
- The challenge of assessing the quality of scientific research in a new era of open access publishing is discussed.
- Antipsychotics: Taking the Long View (August 28, 2013)
- Antipsychotics help people through the crisis of acute psychosis, but the long-term management of chronic mental illness is another matter.
- Infantile Amnesia (August 19, 2013)
- Insights from research into why we do not retain memories from the first four years of life may shed light on the mechanisms underlying learning and memory.
- Healing Invisible Wounds: An Action Plan (August 13, 2013)
- The National Research Action Plan (NRAP) is an effort announced by President Obama aimed at improving prevention, diagnosis, and treatment of mental health conditions and traumatic brain injury in military personnel, veterans, and their families.
- A Sampling of Summer Science (August 6, 2013)
- Intriguing findings published this summer on the genes and disruptions in brain circuitry involved in schizophrenia.
- Getting Serious About Mental Illnesses (July 31, 2013)
- Describes the nuances in using the term “serious mental illness,” and what they might mean for the research that NIMH supports.
- Open Data (June 14, 2013)
- The value of data sharing and collaboration to promote innovation and scientific discovery.
- A National Dialogue (June 4, 2013)
- The President’s launch on June 3 of the National Dialogue on Mental Health, and NIMH’s role in this effort.
Return to Top
VIII. NIMH Science Updates
The latest news and updates from NIMH-supported research:
- NIMH Grantees to Receive 2013 Lasker Award (September 9, 2013)
- NIMH Twitter Chat on Suicide Prevention (September 5, 2013)
- Jane Pearson Talks About Suicide Prevention Research on NPR’s Science Friday (September 3, 2013)
- Webinar on Childhood-Onset Schizophrenia with NIMH’s Judith L. Rapoport, M.D. (August 28, 2013)
- The More Hemispheric Lateralization, the Better Thinking Performance (August 28, 2013)
- Our New Search Engine Delivers Faster, Better Results (August 20, 2013)
- President Obama’s Executive Order Leads to National Research Action Plan (August 13, 2013)
- New Data Reveal Extent of Genetic Overlap Between Major Mental Disorders (August 12, 2013)
- Introduction to RDoC (August 9, 2013)
- Webinar on Ketamine and Next Generation Therapies Featuring NIMH’s Carlos A. Zarate, M.D. (August 6, 2013)
- Stray Prenatal Gene Network Suspected in Schizophrenia (August 1, 2013)
- NAB Unveils Youth Mental Health Awareness Campaign (July 23, 3013)
- Tom Insel Discusses The BRAIN Initiative on The Charlie Rose Brain Series (July 22, 2013)
- Community-based Treatments Offset Depression Disparities (June 25, 2013)
- NIMH Twitter Chat on Post-traumatic Stress Disorder (June 20, 2013)
- Skewed Norms Weaken Case for Early Brain Overgrowth in Autism (June 19, 2013)
- NIH Funds Industry Collaborations to Identify New Uses for Existing Compounds (June 18, 2013)
- Scan Predicts Whether Therapy or Meds Will Best Lift Depression (June 12, 2013)
- Bullying Exerts Psychiatric Effects Into Adulthood (June 11, 2013)
- Our New Look! (June 7, 2013)
Publicizing NIMH research is a communal responsibility. Please help us spread the word about the results of NIMH funding by acknowledging our support of your research, for example, in journal articles (citing your NIMH award by number when possible) and other communications. NIMH has two primary methods of getting the word out: press releases and science updates. All releases and updates are posted to the Science News section of the NIMH Web site. These are also distributed to the public through a mailing list .
If you have a manuscript accepted for publication that describes an especially significant finding, please contact your NIMH Program Official to discuss the possibility of a news release or other forms of dissemination.
Return to Top
IX. Connect with NIMH
Our newest effort to reach our stakeholders is a service that allows you to subscribe for updates sent directly to your email inbox on the NIMH topics of your choice. In addition to our email newsletters and RSS updates, NIMH offers a vodcast series entitled “Speaking of Science,” and YouTube videos on mental health topics. We have also entered the world of Twitter , where we highlight Science Updates, Press Releases, and other timely matters. You can even find us on Facebook !
Inside NIMH is produced by the National Institute of Mental Health. For more information about the Institute, visit our Web site at http://www.nimh.nih.gov. For comments and suggestions about Inside NIMH, please contact the NIMH Webmaster. The material in this newsletter is not copyrighted, and we encourage its use or reprinting.
