2017 Autumn Inside NIMH

Welcome
Welcome to the latest edition of Inside NIMH! We publish Inside NIMH in conjunction with each meeting of the National Advisory Mental Health Council, which advises the Secretary of Health and Human Services (HHS), the Director of the National Institutes of Health (NIH), and the Director of NIMH on all policies and activities relating to the conduct and support of mental health research, research training, and other programs of the Institute. In addition, check out our website for regular updates on timely topics at NIMH. I hope you find this information interesting and helpful. Please let us know if you have questions or comments on this edition.
Sincerely,
Joshua A. Gordon, M.D., Ph.D.
Director, National Institute of Mental Health
If you wish to unsubscribe, subscribe, or change your email address, please contact the NIMH Webmaster or visit the Inside NIMH subscription page .
NIMH Director’s Updates
New and Newsworthy
- NIH Leadership News:
- On June 6, 2017, President Trump announced his selection of Francis Collins, M.D., Ph.D. , to continue as Director of NIH. Dr. Collins has led NIH, the world’s largest supporter of biomedical research, since his initial appointment in August, 2009. He previously served as Director of the National Human Genome Research Institute at NIH from 1993-2008. Dr. Collins led the International Human Genome Project and made landmark discoveries of disease genes.
- On June 9, 2017, President Trump announced his intention to appoint Norman E. “Ned” Sharpless, M.D. , as the new director of the National Cancer Institute (NCI). Dr. Sharpless is Director of the NCI-designated Lineberger Comprehensive Cancer Center at the University of North Carolina, and studies the cell cycle and its role in cancer and aging.
- In October 2017, Josie Briggs, M.D. , will retire from her position as Director of the National Center for Complementary and Integrative Health (NCCIH), after 20 years of service to NIH. Dr. Briggs joined NIH in 1997 as Director of the Division of Kidney, Urologic, and Hematologic Diseases in the National Institute of Diabetes and Digestive and Kidney Diseases, and became Director of NCCIH in 2008. The current NCCIH Deputy Director, David Shurtleff, Ph.D., will serve as Acting Director.
- New Policies and Initiatives:
- Changes to NIMH R21 Applications: NIMH continues to fully support applications for exploratory/developmental research grants (R21s), but is changing how they are accepted (NOT-MH-17-040 ). Effective January 9, 2018, NIMH will no longer accept applications in response to the NIH Parent R21 funding opportunity announcement (FOA; PA-16-161 ). NIMH plans to issue new R21 FOAs in the coming months. For a listing of our currently supported R21 FOAs, see the NIMH Funding Opportunities webpage or the NIH Guide to Grants and Contracts . You can also subscribe to the NIH Guide or to the NIMH Funding Opportunities ListServ for notifications of new announcements, as well as administrative updates and changes to grant policies and procedures. For more information see the NIMH R21 FOAs page.
- Next Generation Researchers Initiative: The 21st Century Cures Act, which became law in December, 2016, instructs NIH to promote policies that encourage researchers to gain earlier independence. At the Advisory Committee to the Director meeting on June 8, 2017, NIH launched the Next Generation Researchers Initiative to enhance stewardship of research dollars and strengthen the biomedical research workforce . This initiative bolsters support for early-stage and mid-career investigators to address longstanding challenges faced by these investigators trying to launch and sustain independent research careers (NOT-OD-17-101 ).
- NIH Clinical Trial Definition and New Requirements: The NIH definition of a clinical trial was revised in 2014, in anticipation of stewardship reforms for clinical trials that will go into effect for competing grant applications and contract proposals received January 25, 2018 and beyond. The definition broadens the scope of applications that will now be labeled as clinical trials. NIH will continue to provide guidance to help researchers understand new clinical trials requirements for grants and contracts, including specific funding opportunities , the new Human Subjects and Clinical Trial form , and requirements for registration and reporting .
- Updated Policy for Certificates of Confidentiality: NIH is updating its policy for issuing certificates of confidentiality. Certificates ensure the privacy of research participants, and the policy applies to all NIH-funded research that involves the collection or use of identifiable, sensitive information about human subjects. Effective October 1, 2017, NIH will automatically provide certificates to any NIH -funded recipients conducting research applicable to this policy (NOT-OD-17-109 ).
- NIH Single Institutional Review Board (IRB) Policy for Multisite Clinical Research: In a recent commentary , Dr. Collins and Carrie Wolinetz, Ph.D., Associate Director for Science Policy, NIH, explain how NIH’s single IRB policy for multisite clinical research is complementary to the final Common Rule . A single IRB review for multi-site studies conducting the same protocol will help streamline the process by eliminating repetition of IRB reviews across sites. The single IRB policy goes into effect on January 25, 2018.
- Updates on Large NIH Longitudinal Studies:
- All of Us Research Program: On June 5, 2017, the NIH All of Us Research Program announced beta testing of its protocols and platforms. The beta phase will include 10,000 to 15,000 participants to inform engagement with diverse communities and improve systems before NIH launches this program nationally. The All of Us Research Program aims to ultimately include one million or more research participants. In addition, on July 25, 2017, the Program announced its first community partner awards .
- Adolescent Brain Cognitive Development (ABCD) Study: The NIH ABCD study , the largest longitudinal study of brain development and child health in the U.S., released its first Fast Track Data on the NIMH Data Archive . The inaugural data release contains unprocessed neuroimaging data and demographics from participants aged 9-10. Qualified researchers may request access through the NIMH Data Archive.
- Interagency Autism Coordinating Committee (IACC) Updates: The IACC met on July 26, 2017 and reviewed the draft IACC Strategic Plan for Autism Spectrum Disorder (ASD), scheduled for publication in Autumn 2017. The updated Strategic Plan includes new objectives for ASD research and service activities, and provides guidance to federal agencies and private partner organizations. For updates on the IACC and autism activities across the community, sign up to receive the new e-newsletter from the NIMH Office of Autism Research Coordination.
- National Advisory Mental Health Council (NAMHC) Workgroup Updates:
- The NAMHC workgroup on Opportunities and Challenges of Developing Information Technologies on Behavioral and Social Science Clinical Research published a report summarizing workgroup discussions and recommendations. The report highlights advances in the field of digital health technology for the assessment, diagnosis, and treatment of mental illnesses, and provides a framework of how these technologies can enable more rapid and nimble research.
- The NAMHC genomics workgroup, charged with advising the NAMHC on future directions in psychiatric genetics and functional genomics, held its second meeting on June 19, 2017. The workgroup is currently drafting a report.
Budget Overview
- FY 2017 Budget: We currently anticipate awarding approximately 565 new and competing research project grants (RPGs) in FY 2017. The projected success rate for competing RPGs in FY 2017 is 21%, a decrease from the FY 2016 success rate of 23%. The projected success rate for Early Stage Investigators (ESIs) is 28%, which is an increase from the FY 2016 success rate of 24%. We anticipate funding a total of 93 new Principal Investigators (PIs) in FY 2017, which is consistent with the number of new PIs we funded in FY 2016.
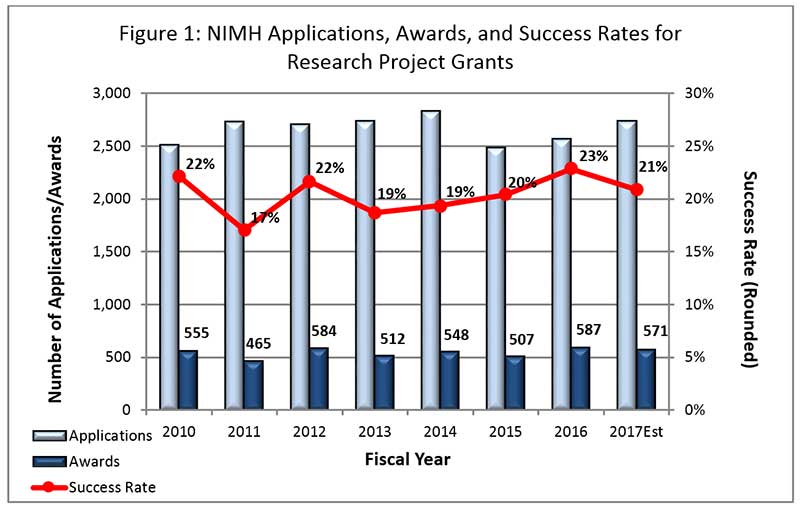
- Outlook for FY 2018: FY 2018 will begin under a continuing resolution (CR). As in the past, while operating under a CR, non-competing grants will be awarded at levels below committed amounts, likely at 90%. As in previous years when operating under a CR, the commitment level for NIMH grants will be determined after we receive a full-year appropriation for FY 2018.
NIMH Staff News and Awards
- Pamela Collins, M.D., M.P.H, transitioned from her position as inaugural Director of the NIMH Office of Research on Disparities and Global Mental Health (ORDGMH) to join the University of Washington as Director of Global Mental Health Research. During her eight-year tenure at NIMH, Dr. Collins spearheaded NIMH research priorities to increase mental health equity in the United States and abroad. Dr. Collins and her team initiated NIMH research and training efforts in global, women’s, and rural mental health research.
- Andrea Beckel-Mitchener, Ph.D., was named Acting Director of ORDGMH. Dr. Beckel-Mitchener has served in several leadership capacities at NIH, including Chief of the Functional Neurogenomics Program in the NIMH Division of Neuroscience and Basic Behavioral Science, Coordinator of the NIH Single Cell Analysis Program, and co-lead of the Cells and Circuits Team for the NIH Brain Research to Advance Innovative Neurotechnologies (BRAIN) Initiative.
- Judith L. Rapoport, M.D., retired from the Division of Intramural Research Programs (IRP) faculty in July 2017 after 41 years of service and continues in the IRP as Scientist Emeritus. Dr. Rapoport was Chief of the Child Psychiatry Branch in the NIMH IRP. She applied a variety of methods to study psychiatric disorders in children, including attention deficit hyperactivity disorder, obsessive-compulsive disorder, and childhood onset schizophrenia.
- We are sad to announce the passing of two remarkable colleagues.
- Howard Eichenbaum, Ph.D., passed away on July 21, 2017. Dr. Eichenbaum served on the NAMHC from 2009-2012, and was Director of Boston University’s Center for Memory and Brain and the Laboratory of Cognitive Neurobiology. Dr. Eichenbaum studied the neuropsychology of memory in animals and the characterization of memory coding properties of neurons.
- Larry Seidman, Ph.D., passed away recently and unexpectedly while attending a meeting on data harmonization in community clinics for first-episode psychosis. A long time NIMH grantee, Dr. Seidman worked as a clinical psychologist at Harvard Medical School and the Massachusetts Mental Health Center/Beth Israel Deaconess Medical Center. He was known for his influential work on the characterization of underlying neuropsychological deficits and assessment of genetic and perinatal risk factors for schizophrenia and co-occurring disorders.
Director’s Highlights: NIMH Scientists and Science
Grantee Awards
NIMH is proud to recognize significant achievement and awards received by our current grantees:
- An NIMH grantee was awarded one of science’s most generous prizes:
Fresenius Prize, Else Kroener Fresenius Foundation- Karl Deisseroth, M.D., Ph.D. (Stanford University)
Notable NIMH Grants
The following is a selection of the Institute’s most recently funded projects that exemplify our efforts to accelerate mental health research and to advance the NIMH Strategic Plan for Research:
- Adolescence is a period of vulnerability for the onset of mental illnesses such as schizophrenia and mood disorders. Beatriz Luna, Ph.D. (University of Pittsburgh) aims to understand brain mechanisms underlying plasticity in cognitive systems during adolescence. Studies will incorporate a variety of neuroimaging techniques to characterize changes in key neurotransmitters, assess structural and functional brain connectivity, and examine higher-order executive functions that develop throughout adolescence. Researchers hope to gain insight into the contributions of different neurotransmitter systems to brain maturational processes during typical development. This knowledge is critical for identifying developmental vulnerabilities that can lead to mental illnesses.
- While electroconvulsive therapy (ECT) can be effective for treating major depressive disorder, adverse cognitive effects and stigma limit its widespread use. An alternative treatment, magnetic seizure therapy (MST), may treat depression at similar rates to ECT, with fewer cognitive side effects (e.g., memory loss). Zafiris Daskalakis, Ph.D. (University of Toronto) seeks to study the safety and efficacy of MST for the treatment of depression in a large, randomized-control study. If MST is found to be effective, this research could impact the treatment of depression.
- Assessing the quality of psychotherapies is crucial to informing evidence-based practices for the treatment of mental illnesses. Investigators are evaluating a complementary range of innovative approaches to assess the quality of psychotherapy across a variety of target populations. Dale Olsen, Ph.D. (SIMmersion, LLC) aims to create a series of web-based virtual client sessions paired with automated scoring algorithms to rate therapists’ cognitive behavioral therapy (CBT) delivery. Shannon Wiltsey Stirman, Ph.D. (Palo Alto Veterans Institute for Research) plans to leverage routinely collected session materials and mobile technology to assess CBT quality for post-traumatic stress disorder, depression, and anxiety disorders. Kristen Benito, Ph.D. (Emma Pendleton Bradley Hospital) seeks to develop a tool to measure and facilitate delivery of therapist-guided exposure treatment for anxiety disorders among youth. Lauren Brookman-Frazee, Ph.D. (University of California San Diego) and Anna Lau, Ph.D. (University of California Los Angeles) plan to develop and validate a toolkit to assess the quality of evidence-based practice delivery for children.
- When taken regularly, HIV pre-exposure prophylaxis (PrEP) provides over 90 percent protection from HIV infection. In a large-scale randomized trial of men who have sex with men in four U.S. cities, Aaron Siegler, Ph.D. (Emory University), and Kenneth Mayer, M.D. (Fenway Health), aim to test a novel home care system designed to improve PrEP adherence and care retention among persons at risk for HIV. The home care system uses a mailed package of assays and online support to reduce the need for in-person PrEP clinical visits. The home care system advances a new paradigm for PrEP clinical care and seeks to strengthen the public health impact of PrEP.
For more information on these and other grants selected for funding, please visit the NIH RePORTER website .
Current Funding Opportunities and Announcements
NIH electronically posts the NIH Guide , a listing of all NIH Funding Opportunity Announcements (FOAs) that includes requests for applications (RFAs), program announcements (PAs), and important notices for the scientific community. Below is a selection of recently issued FOAs in which NIMH participates. The Funding page on the NIMH website has links to listings of all NIMH FOAs and other resources.
You can subscribe to the NIMH Funding Opportunities ListServ to receive the latest information about RFAs and other research funding opportunities from NIMH, as well as administrative updates and changes to grant policies and procedures. You can also subscribe to a separate listserv to receive weekly e-mails from the NIH Guide .
Please refer to a specific FOA for submission instructions including applications due dates, award and eligibility information, agency contacts, and additional information.
NIMH-Administered Requests for Applications
- BRAIN Initiative: Standards to Define Experiments Related to the BRAIN Initiative
- Release date: September 21, 2016; Application due date: October 11, 2017
- R24 announcement (RFA-MH-17-256 )
- BRAIN Initiative: Development and Validation of Novel Tools to Analyze Cell-Specific and Circuit-Specific Processes in the Brain
- Release date: August 11, 2016; Application due date: October 13, 2017
- R01 announcement (RFA-MH-17-220 )
- BRAIN Initiative: Foundations of Non-Invasive Functional Human Brain Imaging and Recording - Bridging Scales and Modalities
- Release date: August 15, 2016; Application due date: October 13, 2017
- R01 announcement (RFA-MH-17-235 )
- BRAIN Initiative: Non-Invasive Neuromodulation - Mechanisms and Dose/Response Relationships for Targeted CNS Effects
- Release date: August 23, 2016; Application due date: October 13, 2017
- R01 announcement (RFA-MH-17-245 )
- BRAIN Initiative: Non-Invasive Neuromodulation - New Tools and Techniques for Spatiotemporal Precision
- Release date: August 23, 2016; Application due date: October 13, 2017
- R01 announcement (RFA-MH-17-240 )
- BRAIN Initiative Cell Census Network (BICCN) - Specialized Collaboratory on Human and Non-Human Primate Brain Cell Atlases
- Release date: October 19, 2016; Application due date: October 13, 2017
- U01 announcement (RFA-MH-17-210 )
- BRAIN Initiative Cell Census Network (BICCN) - Specialized Collaboratory on Mouse Brain Cell Atlas
- Release date: October 19, 2016; Application due date: October 13, 2017
- U01 announcement (RFA-MH-17-230 )
- BRAIN Initiative: Data Archives for the BRAIN Initiative
- Release date: September 21, 2016; Application due date: October 19, 2017
- R24 announcement (RFA-MH-17-255 )
- BRAIN Initiative: Integration and Analysis of BRAIN Initiative Data
- Release date: September 21, 2016; Application due date: October 26, 2017
- R24 announcement (RFA-MH-17-257 )
- Addressing Suicide Research Gaps: Aggregating and Mining Existing Data Sets for Secondary Analyses
- Release date: May 15, 2017; Application due date: November 2, 2017
- R01 announcement (RFA-MH-18-400 )
- Addressing Suicide Research Gaps: Understanding Mortality Outcomes
- Release date: May 26, 2017; Application due date: November 2, 2017
- R01 announcement (RFA-MH-18-410 )
- BRAIN Initiative: Research on the Ethical Implications of Advancements in Neurotechnology and Brain Science
- Release date: August 25, 2017; Application due date: December 7, 2017
- R01 announcement (RFA-MH-18-500 )
- Initiation of a Mental Health Family Navigator Model to Promote Early Access, Engagement and Coordination of Needed Mental Health Services for Children and Adolescents
- Release date: May 2, 2017; Standard due dates apply; Expiration date: January 8, 2018
- R01 announcement (PAR-17-265 )
- R34 announcement (PAR-17-266 )
- Effectiveness Trials for Post-Acute Interventions and Services to Optimize Longer-term Outcomes
- Release date: May 4, 2017; Standard due dates apply; Expiration date: January 24, 2018
- R01 announcement (PAR-17-272 )
- R34 announcement (PAR-17-271 )
- Early Stage Testing of Pharmacologic or Device-based Interventions for the Treatment of Mental Disorders
- Release date: December 13, 2016; Application due dates: October 15, 2017 - October 15, 2018
- R61/R33 announcement (RFA-MH-17-600 )
- R33 announcement (RFA-MH-17-602 )
- Development of Psychosocial Therapeutic and Preventive Interventions for Mental Disorders
- Release date: December 13, 2016; Application due dates: October 17, 2017 - October 15, 2018
- R61/R33 announcement (RFA-MH-17-604 )
- R33 announcement (RFA-MH-17-606 )
- Confirmatory Efficacy Clinical Trials of Non-Pharmacological Interventions for Mental Disorders
- Release date: December 13, 2016; Application due dates: October 17, 2017 - October 15, 2018
- R01 announcement (RFA-MH-17-614 )
- Pilot Effectiveness Trials for Treatment, Preventive and Services Interventions
- Release date: December 13, 2016; Application due dates: October 17, 2017 - October 15, 2018
- R34 announcement (RFA-MH-17-612 )
- Clinical Trials to Test the Effectiveness of Treatment, Preventive, and Services Interventions
- Release date: December 13, 2016; Application due dates: October 17, 2017 - October 15, 2018
- Collaborative R01 announcement (RFA-MH-17-610 )
- R01 announcement (RFA-MH-17-608 )
- BRAIN Initiative: Tools to Facilitate High-Throughput Microconnectivity Analysis
- Release date: August 8, 2016; Application due dates: December 7, 2017 - November 13, 2018
- R01 announcement (RFA-MH-18-505 )
- Reducing the Duration of Untreated Psychosis in the United States
- Release date: May 17, 2016; Standard due dates apply; Expiration date: March 20, 2019
- R01 announcement (PAR-16-265 )
- R34 announcement (PAR-16-264 )
- NIMH Biobehavioral Research Awards for Innovative New Scientists (NIMH BRAINS)
- Release date: February 17, 2017; Application due dates: June 20, 2018 – June 20, 2019
- R01 announcement (RFA-MH-18-200 )
- From Genomic Association to Causation: A Convergent Neuroscience Approach for Integrating Levels of Analysis to Delineate Brain Function in Neuropsychiatry
- Release date: April 11, 2017; Standard due dates apply; Expiration date: September 8, 2020
- R01 announcement (PAR-17-253 )
- Collaborative R01 announcement (PAR-17-252 )
- Innovative Mental Health Services Research Not Involving Clinical Trials
- Release date: April 28, 2017; Standard due dates apply; Expiration date: September 8, 2020
- R01 announcement (PAR-17-264 )
NIMH-Collaborative Requests for Applications
- Intensive Longitudinal Analysis of Health Behaviors: Leveraging New Technologies to Understanding Health Behaviors
- Release date: March 22, 2017; Application due date: September 25, 2017
- U01 announcement (RFA-OD-17-004 )
- U24 announcement (RFA-OD-17-005 )
- BRAIN Initiative: Team-Research BRAIN Circuit Programs - TeamBCP
- Release date: December 2, 2016; Application due date: October 17, 2017
- U19 announcement (RFA-NS-17-018 )
- BRAIN Initiative: Clinical Studies to Advance Next-Generation Invasive Devices for Recording and Modulation in the Human Central Nervous System
- Release date: September 29, 2016; Application due date: October 18, 2017
- UH3 announcement (RFA-NS-17-006 )
- BRAIN Initiative: Next-Generation Invasive Devices for Recording and Modulation in the Human Central Nervous System
- Release date: September 29, 2016; Application due date: October 18, 2017
- U44 announcement (RFA-NS-17-007 )
- UG3/UH3 announcement (RFA-NS-17-005 )
- BRAIN Initiative: New Technologies and Novel Approaches for Large-Scale Recording and Modulation in the Nervous System
- Release date: September 29, 2016; Application due date: October 18, 2017
- U01 announcement (RFA-NS-17-003 )
- BRAIN Initiative: New Concepts and Early - Stage Research for Large - Scale Recording and Modulation in the Nervous System
- Release date: July 17, 2017; Application due date: October 26, 2017
- R21 announcement (RFA-EY-17-002 )
- Silencing of HIV-1 Proviruses
- Release date: May 17, 2017; Application due date: December 6, 2017
- R61/R33 announcement (RFA-AI-17-013 )
- NIH Blueprint for Neuroscience Research: Dynamic Neuroimmune Interactions in the Transition from Normal CNS Function to Disorders
- Release date: August 23, 2017; Application due date: December 7, 2017
- R01 announcement (RFA-AA-18-007 )
- BRAIN Initiative: Optimization of Transformative Technologies for Large Scale Recording and Modulation in the Nervous System
- Release date: September 29, 2016; Application due date: October 18, 2017
- U01 announcement (RFA-NS-17-004 )
- Next Generation Multipurpose Prevention Technologies (NGM)
- Release Date: September 12, 2017; Application due date: March 19, 2018
- R61/R33 announcement (RFA-AI-17-028 )
Future Research Directions
Concept Clearances for Potential New Research Initiatives
This listing of potential future initiatives is meant to provide the earliest possible alert to the field of our research interests and of potential upcoming announcements to solicit that research. While NIMH plans to proceed with these initiatives, their publication and timing are not certain and depend on sufficient funding. The titles and brief descriptions are consistent with the information available at the time of concept clearance. The resultant FOAs may differ from the concepts in the final wording of their titles or other aspects. To send questions about a specific concept, follow the “Submit Comments” link at the bottom of the description.
- Leveraging Electronic Medical Records for Psychiatric Genetic Research
- Novel Approaches to Understanding the Mechanisms of the Neuropsychiatric Symptoms in Alzheimer’s and Advancing Therapy Development
- The NIMH Psychoactive Drug Screening Program (PDSP)
- Paving the Way for Assessing Novel Pediatric Interventions
- Rare Genetic Syndromes as a Window into the Genetic Architecture of Mental Disorders
- A Practice-Based Research Network to Transform Mental Health Care: Science, Service Delivery & Sustainability
- NIMH Career Enhancement Award to Advance Autism Services for Adults and Transition-Age Youth
- Dysregulation and Proximal Risk for Suicide
- Explainable Artificial Intelligence for Decoding and Modulating Behaviorally-Activated Brain Circuits
For more information, please see recent NAMHC-approved concepts, recent public venue-approved concepts, and past NAMHC meetings, which also contains links to meeting agendas, minutes, and Inside NIMH (Director’s Reports).
NIMH-Sponsored Meetings
- HIV Pre-Exposure Prophylaxis (PrEP) Implementation Science Administrative Supplement Meeting: On May 10, 2017, NIMH convened a meeting of researchers who received NIMH administrative supplements for HIV PrEP implementation. These researchers leverage Project PrIDE , a Centers for Disease Control and Prevention initiative to increase PrEP availability and use for individuals at risk for HIV infection. Grantees shared their research progress, strategies to address challenges, and opportunities for collaboration and data harmonization.
- State of Suicide Prevention in Emergency Care: On May 14, 2017, NIMH convened a meeting with federal and private stakeholders to review the current state of knowledge about the scope of the suicide problem in U.S. emergency departments (ED). Specific topics included the risk for morbidity and mortality after an ED visit, effective approaches to risk detection, brief interventions, and follow-up services. Participants also identified research gaps, and discussed policy and practice challenges.
- Autism Spectrum Disorder (ASD) Pediatric, Early Detection, Engagement and Services (ASD PEDS) Network: On June 5-6, 2017, NIMH convened a meeting of grantees funded under the Research on Early Identification and Linkage to Services for ASD FOA (RFA-MH-14-100 ). These investigators work collaboratively to develop and test system-level interventions to universally screen, diagnose, and engage in evidence-based treatment and services children with ASD aged 0-3 years. This meeting provided an opportunity for investigators to present their research, discuss opportunities to leverage data to address the issue of universal screening, and brainstorm shared scientific projects.
- Computational Psychiatry - Opportunities and Challenges for the Future: On June 26-27, 2017, NIMH held a scientific workshop to determine how NIMH can support the development of computational neuroscience approaches to improve the understanding and treatment of mental illnesses. The workshop consisted of breakout sessions that addressed current opportunities and challenges in four key areas; evaluation of the field, computation, psychiatry, and basic science research. A report on the discussions and findings from this workshop is in preparation.
- PsychENCODE Consortium (PEC) Investigators’ Workshop: On July 7, 2017, NIMH hosted the annual PsychENCODE Consortium investigators’ workshop. The PEC is the largest collaborative effort to map genomic regulatory elements across cell types in human brain, and develop molecular models of neuropsychiatric disease. Attendees presented current research findings, discussed data coordination and sharing, publication policies, and future milestones for the consortium.
- Psychiatric Genomics in the Era of Team Science Symposium: On July 10, 2017, NIMH and the Stanley Center for Psychiatric Research led a symposium on the state of the field of neuropsychiatric genomics, including gene discovery, regulation, and computational modeling of disease states. Overarching themes focused on the synergistic nature of research efforts and opportunities to advance the field. Such opportunities include use of large scale, well curated biospecimen and data repositories. A videocast of the event is available.
- Brain Somatic Mosaicism Network (BSMN) Investigators’ Workshop: On July 11, 2017, NIMH held the annual Brain Somatic Mosaicism Network investigators’ workshop. The BSMN conducts whole genome analyses to identify and characterize somatic mosaic variants – the presence of two or more populations of cells with different genotypes – in the human brain. Specifically, the group explores the role of somatic mosaic variants in mental illnesses. Researchers presented updates on their research, discussed data coordination and sharing, publication policies, and future milestones for the network.
- NIMH Outreach Partnership Program: On July 12-14, 2017, NIMH convened the annual meeting of the Outreach Partnership Program. Participants included Outreach Partners from each state, the District of Columbia, and Puerto Rico, National Partners, and NIMH staff. Discussions focused on the NIMH Director’s short-, medium-, and long-term priorities; NIMH-funded Emergency Department-Safety Assessment and Follow-up Evaluation ED-SAFE study; the Ask Suicide-Screening Questions (ASQ) suicide screening questionnaire; the NIH All of Us Research Program; and participation in clinical trials.
- Research Partnerships for Scaling Up Mental Health Interventions in Low- and Middle-Income Countries: On July 31-August 2, 2017, NIMH and the NIMH-funded Strengthening Mental Health and Research Training in Africa (SMART) project hosted the first meeting of the NIMH-funded Scale-Up Hubs in Kampala, Uganda. NIMH established the Scale-Up Hubs to expand evidence-based mental health interventions and build in-country capacity for conducting evidence-based implementation research.
- 2017 Webinar Series on Mental Health Disparities and Global Mental Health Research: NIMH organized two webinar series that ran from August 3-September 13, 2017.
- The mental health disparities webinar, Building Resilience to Reduce Suicide in Arctic Communities showcased two different community-based approaches that aim to build resilience in indigenous communities with the hope of reducing suicide. Presenters highlighted the National Inuit Suicide Prevention Strategy in Canada, and the NIMH Reducing the Incidence of Suicide in Indigenous Groups – Strengths United through Networks (RISING SUN) Initiative.
- The series on global mental health included four webinars. The first webinar, Mental Health Economics: Analyzing Value Date and Time provided an overview of key principles of health economic analysis, methods, and data requirements. The second webinar, Treatment Targets, Target Engagement, and Target Populations in Mental Health Services Research to Improve Public Health: Examples from the Field provided a brief overview of the experimental therapeutics approach to clinical trials and its relevance to public health. The third webinar, Research Capacity Building: Nurturing and Strengthening Emerging Scientists focused on principles and practices for building research capacity in low- and middle-income countries. The final webinar, NIMH Research Domain Criteria (RDoC) initiative served as an introductory review and update on the NIMH RDoC project and the application of RDoC principles in global mental health research.
Electronic Research Administration (eRA) Activities
NIH-Wide Grant News
- NIH Implementation of Final Research Performance Progress Reports (Final RPPR) for Small Business and Innovation Research (SBIR) and Small Business Technology Transfer (STTR) Grants and Cooperative Agreements: Effective June 30, 2017, all SBIR/STTR Phase II final reports must be submitted through the eRA Commons using the Final RPPR rather than the Final Progress Report (NOT-OD-17-085 ).
- Revised Guidance on Salary Limitation for Grants and Cooperative Agreements: This notice makes final what was previously interim guidance. Direct salary support is limited to Executive Level II of the Federal Executive pay scale. The Executive Level II salary was previously set at $185,100, and increased to $187,000 effective January 8, 2017 (NOT-OD-17-087 ).
- NIH Natural Disaster Policy: Hurricanes Harvey and Irma may adversely affect some NIH-applicants’ and recipients’ ability to submit applications or reports on time. When delays occur due to a natural disaster or other emergency, NIH will consider accepting applications late, under specific circumstance, and on a case by case basis (NOT-OD-17-106 and NOT-OD-17-111 ).
For more information on all of these updates, please see the NIH eRA News and Events page .
Questions? Contact the eRA help desk . Note that contacting this help desk is the only way to document problems with an electronic grant application submission. Evidence of this contact is the only way to be eligible for any special consideration by the Center for Scientific Review (CSR) Division of Receipt and Referral, should you run into a system problem with Grants.gov or with eRA that is beyond your control.
Research Training and Career Development
Here is the latest news about Research Training and Career Development at NIMH and NIH:
- Pilot Pediatric Clinical Pharmacology Training Program in Psychiatry: The Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) supports postdoctoral fellows interested in pediatric clinical pharmacology in psychiatry through the T32 Pediatric Clinical and Developmental Pharmacology Training Network . NIMH recently partnered with NICHD to support a trainee at the University of Cincinnati , who will be co-mentored by an pediatric pharmacologist and a child psychiatrist. By participating in this training network, NIMH hopes to increase the number of postdoctoral fellows trained in pediatric clinical pharmacology.
- Opportunity for M.D./Ph.D. Researchers to Gain Research Experience during Clinical Training: NIMH reissued an administrative supplement to provide focused, protected research time for eligible individuals during residency and/or clinical fellowship. Applications are accepted on a continuous basis until April 1 of each year.
Please refer to the NIMH webpage for research training and career development between issues of Inside NIMH to locate the latest news and resources for potential applicants and current awardees.
We are interested in feedback from the community; comments or suggestions related to NIMH’s support for research training and career development may be directed to NIMH_Training@mail.nih.gov. You may also contact NIMH Program Staff with questions or comments.
Director’s Messages
NIMH's Director’s Messages provide insights into the latest topics in mental health research:
- Summer Reading (August 25, 2017): Dr. Gordon offers highlights of his summer reading in science and literature.
- Summer Travels (August 18, 2017): NIMH Director Dr. Joshua Gordon relays highlights of summer scientific meetings that he attended.
- RDoC: Outcomes to Causes and Back (June 22, 2017): Dr. Gordon discusses computational modelling methods that integrate RDoC approaches and clinical diagnostic criteria.
- The Future of RDoC (June 5, 2017): Dr. Gordon talks about using big data approaches to enhance the RDoC research framework.
NIMH Science News
The latest news and updates from NIMH-supported research:
- Depression’s “Transcriptional Signatures” Differ in Men vs. Women (August 28, 2017)
- Scientists Give Star Treatment to Lesser-Known Cells Crucial for Brain Development (August 18, 2017)
- Breakthrough Method Yields Trove of Neuron Subtypes, Gene Regulators (August 10, 2017)
- Mood Stabilizing Medications an Effective Option for Older Adults with Bipolar Disorder (August 7, 2017)
- Patient-Derived Support Cells Stunt Mouse Brain Development (August 7, 2017)
- Our Brains Harbor “Residual Echo” of Neanderthal Genes (July 24, 2017)
- Guidelines Published for Treating PANS/PANDAS (July 21, 2017)
- Imaging Pinpoints Brain Circuits Changed by PTSD Therapy (July 18, 2017)
- Scientists Replay Movie Encoded in DNA (July 12, 2017)
- The NIH NeuroBioBank: Addressing the Urgent Need for Brain Donation (July 7, 2017)
- NIH Names Winners of “Follow that Cell” Phase 2 Competition (June 27, 2017)
- Neuroimaging Technique May Help Predict Autism Among High-Risk Infants (June 16, 2017)
- Brain Circuit Tweak Wins Her Affection (if she’s a vole) (June 13, 2017)
- Connections Strengthen Within Specialized Networks as Brain’s Executive Function Matures (June 1, 2017)
- Pediatrics-based Brief Therapy Outdoes Referral for Youths with Anxiety and Depression (May 31, 2017)
- NIMH Grantee Wins One of Science’s Most Coveted Prizes (May 31, 2017)
- NIMH to Host Multimodal Brain Stimulation Speaker Series (May 24, 2017)
Publicizing NIMH research is a communal responsibility. Please help us spread the word about the results of NIMH funding by acknowledging our support of your research, for example, in journal articles (citing your NIMH award by number when possible) and other communications. NIMH has two primary methods of getting the word out: press releases and science updates. All releases and updates are posted to the Science News section of the NIMH Web site. These are also distributed to the public through a mailing list .
Connect with NIMH
Sign up for the latest mental health news, research advances, upcoming events, publications, clinical trials, meeting summaries, and more. In addition to our email newsletters and RSS updates, please also visit NIMH on Twitter , Facebook , and YouTube , where we highlight Science Updates, Press Releases, and other timely matters.
Inside NIMH is produced by the National Institute of Mental Health. For more information about the Institute, visit our website at https://www.nimh.nih.gov. For comments and suggestions about Inside NIMH, please contact the NIMH Webmaster. The material in this newsletter is not copyrighted, and we encourage its use or reprinting.
