2012 Winter Inside NIMH

Welcome to the latest edition of Inside NIMH. This edition of the newsletter discusses recent funding opportunities and some new initiatives the Institute is considering for the future. We e-publish Inside NIMH after each meeting of the National Advisory Mental Health Council, which advises the Secretary of Health and Human Services; the Director, National Institutes of Health, and the Director of NIMH on all policies and activities relating to the conduct and support of mental health research, research training, and other programs of the Institute. In addition, check out the Director’s blog on our website for regular updates on timely topics at NIMH. I hope you find this information interesting and helpful. Please let us know if you have questions or comments on this edition.
Sincerely,
Thomas R. Insel, M.D.
Director, National Institute of Mental Health
If you wish to unsubscribe, subscribe, or change your email address, please contact the NIMH Webmaster or visit the Inside NIMH subscription page .
I. Message from the NIMH Director
As we settle in to 2012, I would like to update you on a variety of matters related to the NIMH budget, and new activities and plans for the Institute.
Budget Overview
- NIMH awarded 465 new and competing research project grants (RPGs) in FY 2011, the lowest number since 1998 and a 16% reduction from FY 2010.
- This number would have been lower, if not for strategic reductions in numerous areas, including centers, conferences, and our intramural research program.
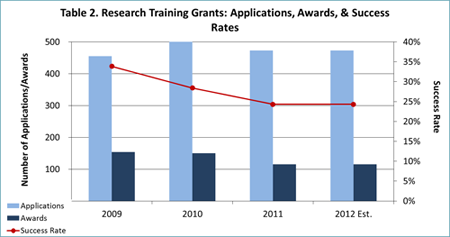
- Thanks largely to these austerity measures, we expect to award over 500 new and competing RPGs in FY 2012 (see Table 1) and over 100 new and competing research training grants (Fs and Ts; see Table 2). These figures are more in line with NIMH’s historical funding patterns than the FY 2011 data are.
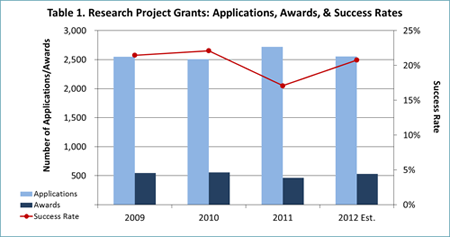
- In FY 2012, NIMH will support new and competing RPGs at a level equivalent to the 15th percentile for established investigators, and at a level equivalent to the 18th percentile for new and early stage investigators. The term “level equivalent” means that some grants under the 15th percentile will not be paid and some higher than this level will be funded, based on programmatic priority and portfolio balance.
- Consistent with new NIH policy, NIMH will discontinue inflationary increases for awards issued in FY 2012.
- The Budget Control Act of 2011 requires the Federal government to reduce spending by $1.5 trillion, with reductions spread evenly over FYs 2013-2021. The method being used to achieve this reduction calls for across-the-board reductions, through a process called sequestration: non-security discretionary programs, including NIH, can expect an approximately 8% reduction in FY 2013 from FY 2011 levels. We don’t know whether additional legislation will change this state of affairs. Stay tuned!
New and Notable
A selection of the Institute’s most recently funded projects that exemplify our efforts to accelerate mental health research and to advance the NIMH Strategic Plan:
- Benjamin Philpot, Ph.D. (University of North Carolina School of Medicine, Chapel Hill) is investigating the use of a class of small molecules that are currently used as cancer therapeutics to restore functionality of a gene, UBE3A, that is effectively deactivated (via epigenetic silencing) in the brain in Angelman’s syndrome, a severe genetic developmental disorder. Dr. Philpot and his colleagues are examining whether their compounds can not only upregulate the silenced gene in a mouse model, but also rescue or reverse the associated physiological and behavioral deficits. Early results were recently published in Nature (Huang et al., 2011 ), suggesting that the team has identified a possible therapeutic strategy for Angelman’s syndrome.
- Vijay Mittal, Ph.D. (University of Colorado at Boulder) is advancing a novel approach to identify an observable biomarker for adolescents at risk for psychosis. He is investigating the structural and functional brain abnormalities that result in characteristic spontaneous movement abnormalities seen in adolescents prior to the onset of schizophrenia. By combining structural MRI, DTI, and quantitative measures of movement, Dr. Mittal and his colleagues will test the hypothesis that atypical development in grey and connective white matter tracts in adolescents in the prodromal phase of schizophrenia contributes to changes in connectivity in the frontal cortex, resulting in movement abnormalities, increased prodromal symptoms, and the onset of psychosis. If successful, this project could lead to earlier interventions that prevent or mitigate the onset of schizophrenia.
- Martha Sajatovic, M.D. (Case Western Reserve University) is testing a model for improving illness self-management among individuals who have both serious mental illness (SMI) and diabetes. In particular, this project is evaluating a psychosocial intervention, Targeted Training in Illness Management, intended to improve mental health symptoms, functioning, and quality-of-life—as well as diabetes health outcomes—in individuals with both SMI and diabetes, compared to treatment as usual. Dr. Sajatovic and her colleagues are conducting the study within a primary care setting at a safety net hospital system. By promoting self-management, Dr. Sajatovic aims to reduce psychiatric symptoms, improve functioning and physical health, and ultimately increase the life-expectancy of individuals with SMI.
For more information on these and other grants selected for funding, please visit the NIH RePORTER website .
- NCATS in FY 2012: As many of you may know, the Director of NIH, Francis Collins, M.D., Ph.D., has asked me to serve as the Acting Director of the new NIH Center, the National Center for Advancing Translational Science (NCATS). Recruitment efforts for a permanent director are underway. I am looking forward to returning full-time to NIMH in the next few months.
- NOT-MH-12-014 : NIMH is offering investigators with active NIMH-supported research grants the opportunity to request one year of supplemental funding in FY 2012. Specific areas of interest have been identified in accordance with the NIMH Strategic Plan.
- We are beginning to discuss updating the Strategic Plan, released in 2008. We will look forward to engaging all of the Institute’s stakeholders to update the Plan.
Return to Top
II. New Announcements about Funding Opportunities
Each week, NIH electronically distributes the NIH GUIDE , a listing of all NIH Funding Opportunity Announcements (FOAs), which include requests for applications (RFAs), program announcements (PAs), and important notices for the scientific community. Below is a selection of recently issued FOAs in which NIMH participates. The Research and Funding page on the NIMH website has links to listings of all NIMH FOAs and other resources.
Note: You can subscribe to the NIMH Funding Opportunities LISTSERV to receive the latest information about RFAs and other research funding opportunities from NIMH, as well as administrative updates and changes to grant policies and procedures. You can also subscribe to a separate LISTSERV to receive weekly emails of the NIH GUIDE .
NIMH-Administered Requests for Applications
Competitive Revision & New Applications for Research on Neural Processes Underlying Sex Differences Related to Risk and Resilience for Mental Illness
This FOA seeks to support revision applications that explicitly test hypotheses regarding neural mechanisms underlying sex differences relevant to mental health. Applications should articulate a strong rationale for how the proposed project will significantly advance our understanding of the etiology and/or underlying pathophysiology of mental disorders in males and females. The R01 and P50 versions of this FOA are limited to revision applications to currently active NIMH-supported R01 and P50 grants. Revision applications will provide funds for 1) recruiting additional subjects to provide enough power to address hypotheses about sex differences or 2) thorough analyses of pre-existing data where sex specific analyses were not initially included, and power is sufficient. The R21 version of this FOA is for new, exploratory grant applications that explicitly test hypotheses regarding neural mechanisms underlying sex differences relevant to mental health.
Release Date: December 13, 2011; Expiration Date: March 14, 2012
- R01 announcement (RFA-MH-13-020 )
- R21 announcement (RFA-MH-13-021 )
- P50 announcement (RFA-MH-13-022 )
New Tools to Study Astrocyte Heterogeneity, Development and Function in Brain Regions Relevant to Mental Illness
This FOA encourages research grant applications that propose the development or adaptation of cutting edge technologies for astrocyte research, discovery-based research on astrocyte diversity, development and/or function in the brain, and the application of these to the study of basic brain processes or pathophysiology relevant to mental illnesses. The primary objective of this FOA is to address barriers to astrocyte research that are due to the scarcity of tools and datasets to target and identify astrocytes rigorously. Applications should aim to transform the field of astrocyte research by generating resources that will be widely used throughout the neuroscience community. Research supported by this initiative will (i) provide new tools for manipulating and identifying astrocytes based on their heterogeneity, developmental stage or functional state; (ii) identify novel phenomic signatures and combinatorial regulatory mechanisms of astrocyte development/function that account for astrocyte diversity; (iii) characterize mechanisms by which astrocytes regulate neural circuits serving cognition, emotion and social function or contribute to abnormal neural function relevant to psychopathology.
Release Date: November 21, 2011; Expiration Date: March 10, 2012
- R01 announcement (RFA-MH-13-010 )
NIMH-Collaborative Requests for Applications
NIH/PEPFAR Collaboration for Advancing Implementation Science in Prevention of Maternal-Child HIV Transmission (PMTCT)
The NIH, in collaboration with the Office of the Global AIDS Coordinator, invites applications for implementation science projects that will inform the President’s Emergency Plan for AIDS Relief (PEPFAR) as they develop more efficient and cost-effective methods to deliver proven interventions for prevention of maternal-child HIV transmission (PMTCT).
Release Date: September 6, 2011; Expiration Date: February 29, 2012
- R01 announcement (RFA-HD-12-210 )
Integrated Preclinical/Clinical Program for HIV Topical Microbicides (IPCP-HTM)
The National Institute of Allergy and Infectious Diseases (NIAID) and NIMH invite applications from single institutions and consortia of institutions to participate in this Funding Opportunity Announcement (FOA), Integrated Preclinical/Clinical Program for HIV Topical Microbicides (IPCP-HTM). The purpose of this FOA is to support integrated and iterative multi-project, multi-disciplinary preclinical and exploratory clinical studies with the goal of advancing a safe, effective and acceptable single or combination topical microbicide for the prevention of the sexual transmission of HIV. A minimum of two research projects and an Administrative Core must be proposed. At least one component (research project or scientific core) must be from a private sector for-profit or not-for-profit company.
Release Date: October 13, 2011; Expiration Date: March 1, 2012
- U19 announcement (RFA-AI-12-003 )
Limited Competition — Women’s Interagency HIV Study (WIHS-V)
The purpose of the Women’s Interagency HIV Study V (WIHS-V) is to characterize the long-term, natural and treated history of HIV infection in the current cohort of women, and recruit and retain new women into the cohort to provide insight into the changing demographics of the HIV epidemic among women in the United States (U.S.) This Limited Competition Funding Opportunity Announcement (FOA) encourages applications from 1) current Clinical Research Sites (CRS), 2) the current Data Management and Analysis Center (WDMAC), and 3 new Clinical Research Site with capacity to enroll targeted populations of women from the Southern region of the U.S. (Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, or Texas only). All cooperative agreement awards funded under this FOA will comprise the WIHS – V epidemiologic cohort.
Release Date: November 29, 2011; Expiration Date: February 23, 2012
- U01 announcement (RFA-AI-12-002 )
Limited Competition: The Medical Education Partnership Initiative Linked Awards (MEPI)
This NIH Funding Opportunity Announcement (FOA), supported by funds from the participating NIH Institutes and Centers (ICs), invites applications from foreign Institutions in Sub-Saharan African countries who are a part of the Medical Education Partnership Initiative Network of institutions to develop research capacity and research education opportunities in priority health areas related to and/or beyond HIV/AIDS. These opportunities are intended to strengthen medical education systems in the countries in which they exist, and build clinical and research capacity in priority health areas related to and/or beyond HIV/AIDS.
Release Date: December 12, 2011; Expiration Date: February 11, 2012
- R25 announcement (RFA-TW-11-004 )
NIH Common Fund Initiatives
The NIH Common Fund encourages collaboration and supports a series of exceptionally high impact, trans-NIH programs. These programs are supported by the Common Fund, and managed by the NIH Office of the Director in partnership with the various NIH Institutes, Centers and Offices. The following projects currently have active funding opportunities and/or notices for NIMH applicants, have already yielded important scientific opportunities for NIMH, or are currently in development:
Molecular Libraries Program
The NIH’s Molecular Libraries Program (MLP) offers access to a growing library of over 370,000 small molecules — chemical compounds that can be used as tools to probe basic biology and advance our understanding of disease. The goal of the MLP is to integrate high-throughput chemical approaches with state-of-the art genetics, cellular, molecular and in vivo biology in a multi-disciplinary effort to discover of proof-of-concept (POC) molecular probes for cell and in vivo systems. Since beginning of 2011, the major focus of MLP centers has been to expedite the discovery and development of high quality probe compounds which will help transform biomedical advances to impact on public health. In addition to meeting the annual goals including implementing 100 HTS and generating 50 small molecule probes, the network centers have been dedicated to share their achievements via peer reviewed literature and productively publish papers for their projects. With more than 400 publications since MLP inception, over 150 manuscripts were published or accepted for publication during 2011. Papers from the centers were the subject of perspectives and reporting in the scientific press, including Nature, Science, and Cell.
Library of Integrated Network-Based Cellular Signatures (LINCS)
The LINCS program supports the high-throughput collection and integrative computational analyses of informative molecular and cellular signatures. A consortium meeting held in October 2011 assembled funded investigators for the first time to address how to integrate multi-dimensional assays into a single coherent LINCS database, to identify promising new assays that could be included in a full-scale LINCS program, to develop new tools to visualize and analyze the complex LINCS database, and to develop strategies to incorporate new expertise and biomedical science areas while also generating a resource of general usefulness to the scientific community.
Single Cell Analysis
NIMH is working with the National Institute for Biomedical Imaging and Bioengineering (NIBIB) to coordinate a new Common Fund program dedicated to Single Cell Analysis . Many biological experiments are performed on groups of cells, under the assumption that all cells of a particular “type” are identical. However, recent evidence reveals that significant heterogeneity exists among individual cells within a population, and these differences can have important consequences for the health and function of the entire population. Experimental approaches that only examine population-level characteristics can obscure these crucial differences. New approaches to single cell analyses are needed to uncover fundamental biological principles and ultimately improve the detection and treatment of disease. Significant challenges currently exist with regard to systematically describing the “state” of a cell, defining normal cell-to-cell variation, measuring the impact of environmental perturbations, understanding cellular responses in the larger context of tissues and networks, and overcoming limitations in measurement approaches. This program seeks to overcome obstacles by supporting the development of innovative tools and analytical approaches and by accelerating the translation and uptake of single cell technology from the bench to the clinic.
Health Economics
The Health Economics Program , launched in the wake of national health care reform, aims to support research on how specific features of the structure or organization of health care delivery organizations and reimbursement systems influence how health care technologies are adopted and combined by health care providers; how they are applied or used for specific patients; and, how those features could be modified to enhance efficiency. The program, co-led by NIMH and NIA, has launched multiple RFAs on the economics of prevention, organization, structure and delivery of care, incentives to incorporate comparative effectiveness results into health care systems, and knowledge to improve long-term care. NIH recently released a Request for Information to solicit input on the feasibility, scope, and design of a State Health Policy Database (SHPD) (NOT-RM-11-019 ) to identify specific policy areas for inclusion in the SHPD, by identifying key research questions that could be addressed using policy information in particular areas, viable research designs that will allow those questions to be answered, and additional data that are required to implement those research designs. Twelve responses were received from researchers in academia and other organizations. This input will assist the Program in developing the SHPDR for scientific research purposes.
Metabolomics Initiative
Metabolomics is the study of low molecular weight molecules or metabolites found within cells and biological systems. The metabolome is a measure of the output of biological pathways and, as such, is often considered more representative of the functional state of a cell than other ‘omics measures such as genomics or proteomics. In addition, metabolites are conserved across various animal species, facilitating the extrapolation of research findings in laboratory animals to humans. Common technologies for measuring the metabolome include mass spectrometry and nuclear magnetic resonance spectroscopy, which can measure hundreds to thousands of unique chemical entities. Despite early promise, challenges remain before the full potential of metabolomics can be realized. Existing metabolomics facilities are at capacity, with relatively few scientists who possess in-depth expertise in metabolomics, and a dearth of training opportunities to gain that expertise. Some companies provide metabolomics services and limited standards; however, issues with cost, intellectual property rights, and limited profit incentives minimize their use in basic, clinical, and translational research. Six Metabolomics RFAs have been issued since mid-November 2011.
NIH Neuroscience Blueprint Initiatives
The Neuroscience Blueprint is a framework to enhance cooperative activities among 16 NIH Institutes, Centers, and Offices that support research on the nervous system. The Blueprint aims to develop research tools, resources, and training and to make them available to the neuroscience community.
Tools to Enhance Studies of Glial Cell Development, Aging, Disease and Repair
The goal of this FOA is to encourage research grant applications that propose to develop or substantially modify existing cutting edge technologies that will advance glial cell research, discovery-based research on glial cell diversity, development and/or function in the central (CNS) and peripheral (PNS) nervous systems. The primary objective of this FOA is to remove barriers to glial cell research that are due to the scarcity of tools, methods and technologies to target and identify glial cells in a rigorous manner. Applications should aim to transform the field of glial cell research by generating tools that will be widely used throughout the neuroscience community. Research supported by this initiative will (i) provide new tools for manipulating and identifying glial cells based on their heterogeneity, developmental stage or functional state; and /or (ii) tools to allow investigation of glial function and processes, thus contributing to our understanding of normal and abnormal neural function.
Release Date: December 21, 2011; Expiration Date: March 30, 2012
- R21 announcement (RFA-HD-12-211 )
Innovative Neuroscience K-12 Education
This initiative encourages Small Business Innovation Research (SBIR) grant applications to develop innovative neuroscience educational tools to be used by or benefit children in kindergarten through 12th grade (K-12). Educational tools can be designed using any media (e.g., paper, electronic, etc.) or format (e.g., simulations, games, videos, notebooks, etc.) for use in or out of school settings, targeting children in groups or alone, with or without adult or teacher participation. Innovative neuroscience educational tools should promote neuroscience knowledge acquisition and application of that knowledge to one’s own life, promote an interest in neuroscience learning and careers, and present a positive and realistic representation of the diversity of people who engage in neuroscience-related research and occupations. Educational tools targeted to increase the diversity of students (i.e., Native American, Black, Hispanic, female, disabled, or otherwise underrepresented) pursuing neuroscience learning are especially encouraged.
Release Date: March 25, 2010; Expiration Date: April 5, 2012
- R43/R44 announcement (PAR-10-054 )
Return to Top
III. Future Research Directions
Concept Clearances for Potential New Research Initiatives
This listing of potential future initiatives is meant to provide the earliest possible alert to the field of our research interests and of potential upcoming announcements to solicit that research. While NIMH plans to proceed with these initiatives, their publication and timing are not certain and depend on sufficient funding. The titles and brief descriptions are consistent with the information available at the time of concept clearance. The resultant FOAs may differ from the concepts in the final wording of their titles or other aspects. To send questions about a specific concept, follow the “Submit Comments” link at the bottom of the description.
- New Experimental Medicine Studies: Fast-Fail Trials (FAST)
- Development of Tools to Explore the Synaptome
- National Center for Advancing Translational Sciences (NCATS) Drug Rescue Program
- A National Neurobiobank
- Eradication of HIV-1 from CNS Reservoirs: Implications for Therapeutics
Related Information
- Recent NAMHC-approved concepts
- Recent public venue-approved concepts
- Past NAMHC meetings (contains links to agendas, minutes, and Director’s Reports)
Summaries of NIMH-Sponsored Scientific Meetings
Research workshops and scientific meetings are some of the best forums in which to identify research gaps and to stimulate new areas of mental health research. Below is a brief description of meetings that NIMH sponsored recently. Questions about a specific meeting can be addressed by the program contact listed in the meeting description. For additional announcements, summaries, agendas, and participant lists from past NIMH-sponsored meetings, conferences, workshops, and lectures, please visit the Scientific Meetings page.
- Post-Traumatic Stress Disorder (PTSD) Risk Prediction
- Closing the Gaps: Reducing Disparities in Mental Health Treatment through Engagement
Return to Top
IV. Update on Electronic Submission of Grant Applications
For more information on all these updates, please see the NIH eRA Submission Items of Interest page .
Application Rejection Messages from Grants.gov
Here are the most common reasons NIH applicants have received the dreaded ‘Rejected with Errors’ message from Grants.gov, in the hope that you can learn from the experience of others and avoid having your applications encounter a similar fate.
Many rejection issues are ‘XML Validation’ or ‘Default Validation Handler’ errors full of techno-babble that would likely require Grants.gov Contact Center assistance to decipher. However, about half of these errors involved the PHS 398 Modular Budget form. The good news is that the current ADOBE-FORMS-B2 application packages include a fix for the underlying cause of many of these errors. If you encounter this type of error, don’t forget to follow our Guidelines for Applicants Experiencing System Issues and document the issue with the help desk in advance of the submission deadline.
The remaining ‘Rejected with Errors’ messages are within your control and would NOT (under most circumstances) be considered ‘System Issues’ allowing for correction after the deadline. So, prevention is your best bet…
- ‘The Closing Date of the grant opportunity for which you have applied has already passed and the grantor agency is no longer accepting applications.’
Grants.gov sends this message under three conditions:- Cause: It is past the Closing/Expiration date of the FOA.
- Prevention: Verify the ‘Expiration Date’ in the FOA text in the NIH Guide for Grants and Contracts or the ‘Current Closing Date for Applications’ in the synopsis on Grants.gov prior to submission.
- Cause: It is past the expiration date of the application package downloaded from Grants.gov and prepared for the submission.
- Prevention: Verify that you are using the correct application package identified by Competition ID. Occasionally, NIH needs to put out new or updated application forms. To transition to new forms, we sometimes post new application packages to an open FOA and expire the old application package. It is possible that the FOA is still active, but the application package used for submission is not. All NIH submissions should be using application packages with Competition IDs of ADOBE-FORMS-B1 or ADOBE-FORMS-B2. Pay attention to NIH Guide notices that talk about form changes (e.g., NOT-OD-11-096 ).
- Cause: The submission is made before the Open date for the FOA.
- Prevention: Check the Open date listed in the FOA text. The Open date is the first date Grants.gov will accept applications for the opportunity. In most cases, NIH will set the Open Date to 30 days before the first submission deadline. It’s not just a grant-land myth…there are applicants that submit more than 30 days in advance. It doesn’t happen often, but when it does it is a confusing and unfortunate error.
- Cause: It is past the Closing/Expiration date of the FOA.
- ‘You are not designated by your organization to be an Authorized Organizational Representative and your application cannot be validated…’
- Cause: You are not an authorized AOR for the DUNS number included on the application.
- Prevention: Verify that the DUNS number included in the application is correct and matches the DUNS number in your Grants.gov applicant profile. Also, you should see “AOR Status: Approved” near the top of the left navigation when you login to the Grants.gov Applicant Center. If you don’t, your organizations E-Biz POC will need to assign you the AOR privilege.
- Cause: You are not an authorized AOR for the DUNS number included on the application.
- ‘This application contains an attachment(s) with a filename that does not meet Grants.gov requirements. To ensure that your application package will be successfully submitted to the grantor agency please adhere to the following guidelines: avoid using special characters (example: &,-,*,%,/,#) in attachment file names (including periods (.)), attaching documents with the same file name and limit the file name to 50 characters or less.’
- Cause & Prevention: The message pretty much says it all. In addition to Grants.gov’s advice, system-to-system submitters should make sure all attachment filenames are unique within the application. Grants.gov doesn’t give an error for that one, but eRA does (see known eSubmission system issues ).
Return to Top
V. Research Training and Career Development
Request for Information (RFI): Input into the Deliberations of the Advisory Committee to the NIH Director Working Group on Diversity in the Biomedical Research Workforce
This Notice is a time-sensitive Request for Information (RFI) requesting input into the deliberations of the Advisory Committee to the NIH Director (ACD) Working Group on Diversity in the Biomedical Research Workforce. The Working Group would like to gather input from various sources, including extramural and intramural researchers, academic institutions, industry, and the public, to help inform the development of recommendations to present to the ACD and the NIH Director on actions the NIH can take to increase the diversity of the biomedical research workforce.
Release Date: January 10, 2012; Response Date: February 24, 2012
- RFI Notice: NOT-OD-12-031
We’re interested in feedback from the community; comments or suggestions related to NIMH’s support for research training and career development may be directed to NIMH_Training@mail.nih.gov.
Return to Top
VI. Recent NIMH Science News
The latest news and updates from NIMH-supported research:
- Ethnic Disparities Persist in Depression Diagnosis and Treatment Among Older Americans (January 26, 2012)
- Atypical Antipsychotic More Effective than Older Drugs in Treating Childhood Mania, but Side Effects Can Be Serious (January 11, 2012)
- Turning on Dormant Gene May Hold Key for Correcting a Neurodevelopmental Defect (January 5, 2012)
- NDAR Federation Creates Largest Source of Autism Research Data to Date (December 12, 2011)
- Suspect Gene Variants Boost PTSD Risk after Mass Shooting (December 1, 2011)
- HIV Variants in Spinal Fluid May Hold Clues in Development of HIV-related Dementia (November 30, 2011)
- Training Peers Improves Social Outcomes for Some Kids with ASD (November 28, 2011)
- Neurons Grown from Skin Cells May Hold Clues to Autism (November 28, 2011)
- Interventions Show Promise in Treating Depression Among Preschoolers (November 17, 2011)
- Widely Used Screening Tool Shown to Successfully Predict Suicide Attempts (November 10, 2011)
- Our Brains Are Made of the Same Stuff, Despite DNA Differences (October 26, 2011)
- Perinatal Antidepressant Stunts Brain Development in Rats (October 24, 2011)
- National Survey Dispels Notion that Social Phobia is the Same as Shyness (October 17, 2011)
- Brain Chemical Linked to Joylessness Provides Insight Into Teen Depression (October 6, 2011)
- Prescribed stimulant use for ADHD continues to rise steadily (September 28, 2011)
Publicizing NIMH research is a communal responsibility — we need your help! Please help us spread the word about the results of NIMH funding by acknowledging our support of your research, for example, in journal articles (citing your NIMH award by number when possible) and other communications. NIMH has two primary methods of getting the word out: press releases and science updates. All releases and updates are posted to the Science News section of the NIMH Website.
If you have a manuscript accepted for publication that describes an especially significant finding, please contact your NIMH program director to discuss the possibility of a news release or other forms of dissemination.
Return to Top
VII. Connect with NIMH
Our newest effort to reach our stakeholders is a service that allows you to subscribe for updates sent directly to your email inbox on the NIMH topics of your choice. In addition to our email newsletters and RSS updates, NIMH offers audio pieces and videos about mental health topics, and has its own YouTube channel . We have also entered the world of Twitter , where we highlight Science Updates, Press Releases, and other timely matters. You can even find us on Facebook ! Be sure to read our Director’s Blog for insights into the latest topics in mental health research.
Check us out!
