2015 Winter Inside NIMH

Welcome to the latest edition of Inside NIMH. We publish Inside NIMH in conjunction with each meeting of the National Advisory Mental Health Council, which advises the Secretary of Health and Human Services, the Director of the National Institutes of Health, and the Director of NIMH on all policies and activities relating to the conduct and support of mental health research, research training, and other programs of the Institute. In addition, check out the NIMH Director’s Blog website for regular updates on timely topics at NIMH. I hope you find this information interesting and helpful. Please let us know if you have questions or comments on this edition.
Sincerely,
Tom Insel, MD
Director, National Institute of Mental Health
If you wish to unsubscribe, subscribe, or change your e-mail address, please contact the NIMH Webmaster or visit the Inside NIMH subscription page .
Winter 2015 Contents
- Message from the NIMH Director
- Director’s Highlights: NIMH Scientists and Science
- New Announcements about Funding Opportunities
- Future Research Directions
- Update on Electronic Research Administration (eRA) Activities
- Research Training and Career Development
- Director’s Blog
- NIMH Science Updates
- Connect with NIMH
I. Message from the NIMH Director
A wintry welcome from NIMH, and we have much to discuss! We are releasing the new, 2015 NIMH Strategic Plan, soliciting new grants via the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative promoting guidelines to enhance the reproducibility and validity of clinical trials, clarifying expectations for data sharing, and much more.
News to Know
- 2015 NIMH Strategic Plan for Research: NIMH anticipates publishing the new, 2015 NIMH Strategic Plan for Research by March 2015. This revised plan has been reviewed by our National Advisory Mental Health Council (NAMHC) and informed by extensive public comment. The February 2015 NAMHC meeting will feature the final round of review and discussion. Although the overarching strategic objectives have not changed substantially, the scientific landscape, technological possibilities, and the public health context have all evolved since the Plan was first released in 2008. As with the 2008 Strategic Plan, the 2015 edition will be supplemented by an array of Strategic Research Priorities that concretize how we aim to meet our objectives, and how the scientific community can partner with us to achieve our goals. We look forward to sharing the Plan with you.
- BRAIN Initiative: On September 30, 2014, NIH announced its first wave of grants in support of the BRAIN Initiative. This initial round of awards—to more than 100 investigators in 15 states and several countries—totaled $46 million to support the goal of developing deeper understanding of the brain that will ultimately catalyze new treatments and cures for devastating brain disorders and diseases. A second round of funding announcements , including several announcements led by NIMH (see the New Announcements about Funding Opportunities section below), were released in November and December 2014. For more information about NIMH-led BRAIN Initiative announcements, please contact BRAIN-info-NIMH@mail.nih.gov.
- Enhancing Diversity in the Biomedical Workforce: In October 2014, NIH announced the award of nearly $31 million in fiscal year (FY) 2014 funds to develop new approaches that engage researchers, including those from backgrounds underrepresented in biomedical sciences, and prepare them to thrive in the NIH-funded workforce. These awards are part of a projected five-year program to support more than 50 awardees and partnering institutions in establishing a national consortium to develop, implement, and evaluate approaches to encourage individuals to start and stay in biomedical research careers. Supported by the NIH Common Fund and all 27 NIH Institutes and Centers, 12 awards will be issued as part of three initiatives of the Enhancing the Diversity of the NIH-Funded Workforce program .
- New Inclusion Management System (IMS): In October 2014, NIH released guidance to notify NIH applicants and grantees about using the new IMS for reporting sex/gender, race, and ethnicity information as required by the NIH Policy on the Inclusion of Women and Minorities in Clinical Research (NOT-OD-15-005 ). This new IMS comes on the heels of a recent NIH Request for Information concerning the agency’s intention to develop and implement policies to require applicants to consider sex as a biological variable in the design and analysis of NIH-funded research involving animals and cells (NOT-OD-14-128 ). NIH anticipates that such policies will aid in improving the quality and reproducibility of research findings.
Clinical Trials Policy Updates
- Use of a Single Institutional Review Board (IRB) for Multi-site Studies: In December 2014, NIH issued a draft policy (NOT-15-026 ) for the use of single IRBs in multi-site clinical research studies. When regulations for the protection of human subjects were first published, most clinical research was conducted at a single institution. Since then, the research landscape has evolved, and many studies are carried out at multiple sites and within large networks. Multi-site studies often are able to recruit more individuals from diverse populations and generate important results in less time. However, working through IRB review at each site can add delay without increasing the ethical protections for the research participants in the study. NIH is proposing a policy change such that all NIH-funded multi-site studies carried out in the U.S., whether supported through grants, contracts, or in the NIH intramural program, should use a single IRB. Exceptions to the policy will be allowed if local IRB review is necessary to meet the needs of specific populations or where it is required by federal, tribal, or state laws or regulations. The public comment period for the proposed policy closed on January 29, 2015.
- Enhancing the Transparency of Clinical Trials HHS-wide: In November 2014, HHS issued a Notice of Proposed Rulemaking (NPRM) , which proposes regulations to implement reporting requirements for clinical trials that are subject to Title VIII of the Food and Drug Administration Amendments Act of 2007 (FDAAA). The proposed rule clarifies requirements to clinical researchers for registering clinical trials and submitting summary trial results information to ClinicalTrials.gov . Importantly, the NPRM proposes expanding the scope of clinical trials required to submit summary results to include trials of unapproved, unlicensed, and uncleared products. In addition, NIH has proposed a policy to promote transparency for all NIH-funded clinical trials, whether or not they are subject to FDAAA. The proposed policy expects registration and submission of results information like that required by FDAAA in ClinicalTrials.gov of every clinical trial that receives NIH funds.
- Common Data Elements and Data Sharing: NIMH’s own new policy regarding clinical trials related to mental illnesses (NOT-MH-14-015 ) goes further than the proposed NIH policy on transparency by (a) focusing on individual level data, and (b) expecting data sharing at regular intervals during the trial, not just at the conclusion of the project. NIMH-funded scientists involved in clinical trials are expected to enter individual level data into our National Database for Clinical Trials Related to Mental Illness (NDCT). At the same time, NIMH is developing common data elements with the community to ensure that trials can be compared with fidelity. The importance of having individual-level data to share became apparent in a new paper of 37 reanalyses of published clinical trials. In 35% of the clinical trials, the reanalysis led to a different interpretation than in the original paper, with implications for the types and numbers of patients who should be treated.
- Recruitment and Monitoring. The Institute is in the process of formulating its own additional policies regarding the protection of human study participants in NIMH-funded clinical trials—in particular, policies regarding reporting of recruitment milestones and Data Safety and Monitoring Board governance. Please see our Web site for the most up-to-date guidance on risk-based monitoring.
Budget Overview
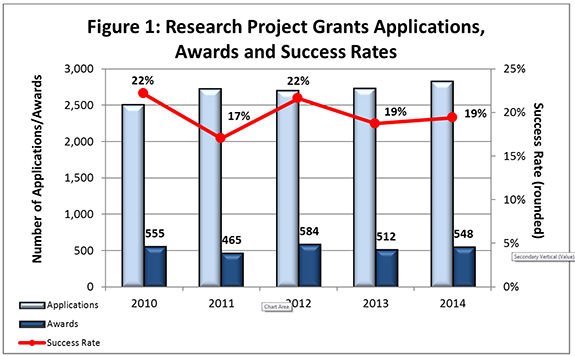
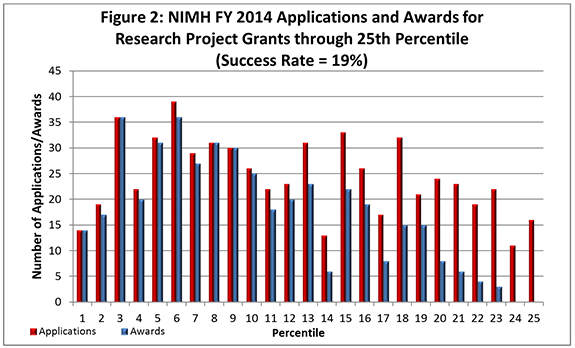
- FY 2014 Budget: NIMH awarded 548 new and competing research project grants (RPGs) in 2014 and achieved an overall success rate of 19% (defined as number of RPG applications funded divided by the number of applications received; see Figures 1 and 2 (note that in Figure 2, the total number of funded grants do not add up to 548, as not all grants are percentiled)). This level represents an increase of 36 awards above the 512 RPGs awarded in FY 2013. With 18 of the new awards funded via the BRAIN Initiative, the number of overall awards is comparable with the FY 2010-2013 average of 529 awards. NIMH awarded grants to 96 New Principal Investigators, and achieved a success rate o575f 23% for Early Stage Investigators (ESIs).
- Figure 3 (below) shows the NIMH budget in appropriated (current) versus constant (FY 1998) dollars. Constant dollars are “inflation adjusted” for variations in the purchasing power of the dollar over time. Dollar amounts are adjusted based on the Biomedical Research and Development Price Index (BRDPI). The annual change in BRDPI indicates how much the NIH budget must change to maintain purchasing power similar to that in FY 1998.
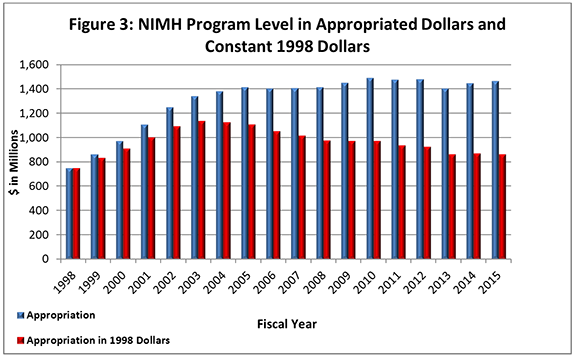
- FY 2015 Budget:On December 16, 2014, President Obama signed an omnibus appropriation (Public Law 113-235) that funds NIH, along with most of the Federal government, through September 30, 2015. The amount provided to NIMH, $1.463 billion, represents a $16.9 million (1.2%) increase over the NIMH FY 2014 Appropriation, but still represents a $17.2 million reduction below the FY 2012 Appropriation. NIMH’s FY 2015 appropriation includes an increase in funding of $12.4 million intended for the President’s BRAIN Initiative .
- Prior to the passing of the appropriation, NIH had been operating under a Continuing Resolution (CR). During the CR period, NIMH issued non-competing research grant awards at a level below that indicated on the most recent Notices of Award (generally up to 90% of the previously committed level). As in previous years, NIMH will adjust these awards to restore them to their committed levels.
Program Updates
- The Research Domain Criteria (RDoC) project has grown into a significant cross-cutting effort for NIMH—so much so that the Institute has created a new RDoC Unit within the Office of the NIMH Director, with Bruce Cuthbert, PhD, at the helm. The RDoC Unit will be dedicated to furthering the goals of the RDoC project, and to overseeing various activities of the project’s day-to-day functioning, including the RDoC Database (RDoCdb) and the RDoC Discussion Forum, a soon-to-be-launched online platform where investigators and clinicians can converse and collaborate with each other around the RDoC framework in a virtual environment.
- On October 2, 2014, NIMH’s Office of Constituency Relations and Public Liaison (OCRPL) and Office for Research on Disparities and Global Mental Health (ORDGMH) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development entered into a two-year collaboration with Delta Sigma Theta Sorority, Inc. (DST) called the Mental Health Across the Lifespan Initiative. The educational outreach initiative seeks to raise awareness about certain mental health conditions affecting women and their families including postpartum depression, bullying, and successful aging later in life. This initiative will harness the power of the organization’s membership network to extend the reach of NIH’s research-based information directly into the communities served by more than 1,000 DST chapters in the U.S. and abroad.
NIMH Staff Awards
- American Psychological Association, Meritorious Research Service Commendation:
- LeShawndra Price, PhD, Chief, Research Scientist Development Program, ORDGMH, for identifying research priorities for federal funding and designing funding initiatives, supporting the implementation of research projects, nurturing junior and mid-career scientists, coordinating efforts to increase diversity in the scientific workforce, and supporting research which recognizes the needs of minority and underserved populations.
- Society for Neuroscience, Julius Axelrod Prize:
- Susan G. Amara, PhD, Director of the Division of Intramural Research Programs, for career-long research with an enduring impact in both basic and clinical neuropharmacology, and for exemplary mentoring of a large number of neuroscientists, many of whom have become leaders of their respective fields.
Return to Top
II. Director’s Highlights: NIMH Scientists and Science
Grantee Awards
NIMH is proud to recognize significant awards received by our current grantees:
- American College of Neuropsychopharmacology, Daniel H. Efron Research Award:
- Sheena Josselyn, PhD (Unversity of Toronto)
- Association for Psychological Science, Janet Taylor Spence Award & F. J. McGuigan Early Career Investigator Prize:
- Leah Somerville, PhD (Harvard University)
- Institute of Medicine, Rhoda and Bernard Sarnat International Price in Mental Health:
- Vikram Patel, MSc, PhD (London School of Hygiene and Tropical Medicine)
Notable NIMH Grants
Here is a selection of the Institute’s most recently funded projects that exemplify our efforts to accelerate mental health research and to advance the NIMH Strategic Plan:
- Anxiety is a common mental health problem among youth, but most anxious teens do not receive appropriate care. A number of small studies have indicated that computer-delivered, cognitive-bias–modification interventions can change attentional biases toward threat-related stimuli and lead to clinical benefit. Gregory Clarke, PhD (Kaiser Foundation Research Institute), is spearheading an innovative study to collect definitive data regarding the utility of Cognitive Bias Modification (CBM) for treating anxiety among youth using a large, diverse sample accrued via the Kaiser Permanente Health Care Network. The enrolled youth will use a self-administered version of CBM that is downloaded and installed on their home computers. This trial will serve the dual aims of (1) efficiently providing data on CBM from nearly 500 youth who vary in terms of anxiety severity and other potential moderating factors; and (2) demonstrating the feasibility of using a health care network platform for efficiently identifying study participants and testing the utility of a psychosocial intervention. Thus, this project has the potential to efficiently yield data on a low-cost, accessible, and easy-to-disseminate treatment that has few of the barriers to uptake associated with other existing evidence-based approaches.
- Childhood trauma is strongly associated with the development of mental illnesses later in life; however, the mechanisms that link these phenomena are unknown. Katie McLaughlin, PhD (University of Washington), has proposed that early adverse experiences disrupt brain development in regions associated with emotion regulation, and that these early disruptions in the development of brain structure and function serve to increase risk for later mental illnesses. To test this hypothesis, Dr. McLaughlin and her research team are recruiting children, aged 8-16 years, with and without a history of traumatic experiences, for a brain imaging study. The researchers will assess the strength of connectivity between the amygdala and the prefrontal cortex in these children, as a function of frequency and severity of exposure to trauma during childhood. Ultimately, the findings gleaned from this study may inform prevention efforts aimed at children who experience trauma.
- How do the many areas of the brain coordinate their activity in an orderly way without producing chaotic brain signals, uninterpretable perceptions, or disordered thinking? Possibly, the brain synchronizes the activity of two or more regions that need to communicate information at a given moment, to allow separate communication “channels” at different synchronous frequencies. Laura Colgin, PhD (University of Texas at Austin), is testing the hypothesis that two such channels, fast gamma (~65-100 Hz) and slow gamma (~25-55 Hz), are associated with different types of memory processing in the hippocampus and entorhinal cortex. Dr. Colgin will test the hypothesis that fast gamma couples the hippocampus with medial entorhinal cortex for the transmission of an animal’s current location, in order to encode memories that have just occurred in the recent past. Slow gamma, on the other hand, is hypothesized to couple preferentially hippocampal sub-areas CA1 and CA3 in order to plan future paths. In this way, the two separate frequencies might be encoding past versus future events. Furthermore, a translational component of this study will test whether electrical stimulation at slow or fast gamma can enhance memory encoding versus retrieval, and whether these deficits can be corrected in a rat model of Fragile X syndrome.
For more information on these and other grants selected for funding, please visit the NIH RePORTER website .
Return to Top
III. New Announcements about Funding Opportunities
Each week, NIH electronically distributes the NIH GUIDE , a listing of all NIH Funding Opportunity Announcements (FOAs) that include requests for applications (RFAs), program announcements (PAs), and important notices for the scientific community. Below is a selection of recently issued FOAs in which NIMH participates. The Funding page on the NIMH website has links to listings of all NIMH FOAs and other resources.
Note: You can subscribe to the NIMH Funding Opportunities ListServ to receive the latest information about RFAs and other research funding opportunities from NIMH, as well as administrative updates and changes to grant policies and procedures. You can also subscribe to a separate listserv to receive weekly e-mails of the NIH GUIDE .
NIMH-Administered Requests for Applications
- Pilot Effectiveness Studies and Services Research Grants
- Release date: February 24, 2014; Application due date: February 18, 2015
- R34 announcement (RFA-MH-15-330 )
- Clinical Trials to Test the Effectiveness of Treatment, Preventive, and Services Interventions
- Release date: February 24, 2014; Application due date: February 18, 2015
- R01 announcement (RFA-MH-15-320 )
- Exploratory Clinical Trials of Novel Interventions for Mental Disorders
- Release date: February 24, 2014; Application due date: February 18, 2015
- R33 announcement (RFA-MH-15-310 )
- Exploratory Clinical Trials of Novel Interventions for Mental Disorders
- Release date: February 24, 2014; Application due date: February 18, 2015
- R21/R33 announcement (RFA-MH-15-300 )
- Confirmatory Efficacy Clinical Trials of Non-Pharmacological Interventions for Mental Disorders
- Release date: May 14, 2014; Application due date: February 19, 2015
- R01 announcement (RFA-MH-15-340 )
- BRAIN Initiative: Development and Validation of Novel Tools to Analyze Cell-Specific and Circuit-Specific Processes in the Brain
- Release date: November 18, 2014; Application due date: March 19, 2015
- U01 announcement (RFA-MH-15-225 )
- BRAIN Initiative: Short Courses in Research Tools and Methods
- Release date: December 19, 2014; Application due date: March 19, 2015
- R25 announcement (RFA-MH-15-220 )
- BRAIN Initiative: Short Courses in Computational Neuroscience
- Release date: December 19, 2014; Application due date: March 19, 2015
- R25 announcement (RFA-MH-15-215 )
- BRAIN Initiative: Planning for Next Generation Human Brain Imaging
- Release date: December 19, 2014; Application due date: March 19, 2015
- R24 announcement (RFA-MH-15-200 )
- Novel Assays to Address Translational Gaps in Treatment Development
- Release date: January 12, 2015; Application due date: April 4, 2015
- UH2/UH3 announcement (RFA-MH-16-220 )
- NIMH Biobehavioral Research Awards for Innovative New Scientists (NIMH BRAINS)
- Release date: June 11, 2014; Application due dates: October 23, 2015; October 24, 2016
- R01 announcement (RFA-MH-15-600 )
NIMH-Collaborative Requests for Applications
- BRAIN Initiative: Integrated Approaches to Understanding Circuit Function in the Nervous System
- Release date: November 5, 2014; Application due date: February 11, 2015
- U01 announcement (RFA-NS-15-005 )
- BRAIN Initiative: Optimization of Transformative Technologies for Large Scale Recording and Modulation in the Nervous System
- Release date: November 5, 2014; Application due date: February 11, 2015
- U01 announcement (RFA-NS-15-004 )
- BRAIN Initiative: New Technologies and Novel Approaches for Large-Scale Recording and Modulation in the Nervous System
- Release date: November 5, 2014; Application due date: February 11, 2015
- U01 announcement (RFA-NS-15-003 )
- Nuclear Organization and Function Interdisciplinary Consortium
- Release date: December 23, 2014; Application due date: February 24, 2015
- U54 announcement (RFA-RM-14-030 )
- NIH Big Data to Knowledge (BD2K) Initiative Research Education: Open Educational Resources for Sharing, Annotating and Curating Biomedical Big Data
- Release date: November 26, 2014; Application due date: March 18, 2015
- R25 announcement (RFA-LM-15-002 )
- NIH Big Data to Knowledge (BD2K) Initiative Research Education: Massive Open Online Course (MOOC) on Data Management for Biomedical Big Data
- Release date: November 26, 2014; Application due date: March 18, 2015
- R25 announcement (RFA-LM-15-001 )
- NIH Big Data to Knowledge (BD2K) Biomedical Data Science Training Coordination Center
- Release date: December 19, 2014; Application due date: March 18, 2015
- U24 announcement (RFA-ES-15-004 )
- NIH Big Data to Knowledge (BD2K) Enhancing Diversity in Biomedical Data Science
- Release date: January 12, 2015; Application due date: March 20, 2015
- R25 announcement (RFA-MD-15-005 )
- Science of Behavior Change: Assay Development and Validation for Self-Regulation Targets
- Release date: January 8, 2015; Application due date: March 21, 2015
- UH2/UH3 announcement (RFA-RM-14-020 )
- Science of Behavior Change: Assay Development and Validation for Stress Reactivity and Stress Resilience Targets
- Release date: January 8, 2015; Application due date: March 21, 2015
- UH2/UH3 announcement (RFA-RM-14-019 )
- Science of Behavior Change: Assay Development and Validation for Interpersonal and Social Processes Targets
- Release date: January 8, 2015; Application due date: March 21, 2015
- UH2/UH3 announcement (RFA-RM-14-018 )
- NIH Science of Behavior Change Resource and Coordinating Center
- Release date: January 8, 2015; Application due date: March 21, 2015
- U24 announcement (RFA-RM-14-017 )
- Innovative Measures of Oral Medication Adherence for HIV Treatment and Prevention
- Release date: December 1, 2014; Application due date: March 26, 2015
- R01 announcement (RFA-AI-14-071 )
- Mentored Career Development Award in Biomedical Big Data Science for Clinicians and Doctorally Prepared Scientists
- Release date: January 15, 2015; Application due date: April 2, 2015
- K01 announcement (RFA-HG-14-007 )
- Metabolomics Core for the Undiagnosed Diseases Network
- Release date: January 21, 2015; Application due date: April 15, 2015
- U01 announcement (RFA-RM-15-001 )
- Predoctoral Training in Biomedical Big Data Science
- Release date: April 22, 2014; Application due date: July 28, 2015
- T32 announcement (RFA-HG-14-004 )
- NIH Pioneer Award Program
- Release date: August 8, 2013; October 10, 2015
- DP1 announcement (RFA-RM-13-006 )
- NIH Director's New Innovator Award Program
- Release date: August 8, 2013; Application due date: October 17, 2015
- DP2 announcement (RFA-RM-13-007 )
- Open Educational Resources for Biomedical Big Data
- Release date: January 16, 2014; Application due date: April 2, 2016
- R25 announcement (RFA-HG-14-009 )
- Courses for Skills Development in Biomedical Big Data Science
- Release date: January 16, 2014; Application due date: April 2, 2016
- R25 announcement (RFA-HG-14-008 )
- Revisions to Add Biomedical Big Data Training to Active Institutional Training Grants
- Release date: April 22, 2014; Application due date: July 29, 2016
- T32 announcement (RFA-HG-14-005 )
Return to Top
IV. Future Research Directions
Concept Clearances for Potential New Research Initiatives
This listing of potential future initiatives is meant to provide the earliest possible alert to the field of our research interests and of potential upcoming announcements to solicit that research. While NIMH plans to proceed with these initiatives, their publication and timing are not certain and depend on sufficient funding. The titles and brief descriptions are consistent with the information available at the time of concept clearance. The resultant FOAs may differ from the concepts in the final wording of their titles or other aspects. To send questions about a specific concept, follow the "Submit Comments" link at the bottom of the description.
- Going to Scale with Mental Health Innovations in Low- and Middle-Income Countries
- Lifespan Human Connectome Project: Children and Adolescents
- Adaptation/Optimization of Technology (ADOPTech) to Support Social Functioning
For more information, please see recent NAMHC-approved concepts, recent public venue-approved concepts, and past NAMHC meetings, which also contains links to meeting agendas, minutes, and Inside NIMH (Director’s Reports).
NIMH-Sponsored Meetings
- Biobehavioral Research Awards for Innovative New Scientists (BRAINS) Award Ceremony: In November 2014, NIMH held a congratulatory ceremony to honor the eight FY 2014 awardees of its Biobehavioral Research Awards for Innovative New Scientists (BRAINS) program. The BRAINS program supports highly creative and promising junior scientists who are committed to enabling NIMH to fulfill its mission with innovative, ground-breaking, and potentially risky research approaches to transform the understanding and treatment of mental illnesses. The ceremony was held in conjunction the annual Society for Neuroscience meeting in Washington, DC. Each BRAINS investigator gave a brief overview of their project funded through the program. NIMH leadership, program staff, and previous BRAINS award recipients attended. The BRAINS RFA has been reissued for 2015 through 2017. The 2014 Awardees are:
- Susanne Elizabeth Ahmari, MD, PhD (University of Pittsburgh):Testing the Role of Circuit Plasticity in the Pathology and Treatment of Abnormal Repetitive Behaviors
- Jed Thomas Elison, PhD (University of Minnesota): Infant Brain and Behavioral Signatures of Later Emerging Risk for Psychopathology
- Zachary Aaron Kaminsky, PhD (Johns Hopkins University): Neuroimaging epigenetics of prospective postpartum depression biomarkers
- Natalia M. Kleinhans, PhD (University of Washington): Molecular Mechanisms of Atypical Habituation in Autism Spectrum Disorders
- Zhongming Liu, PhD (Purdue University West Lafayette): Multimodal Hyperspectral Imaging of Brain Activity and Connectivity
- Daniel Mamah, MD (Washington University): Connectomics in Psychiatric Classification
- Amar Sahay, PhD (Massachusetts General Hospital): Molecular control of excitation-inhibition balance to encode ambiguous threats
- Jared William Young, PhD (University of California San Diego): A model organism of brain circuitry and behavioral switching for bipolar disorder
- NIMH Alliance for Research Progress Fall Meeting: On October 14, 2014, the NIMH Office of Constituency Relations and Public Liaison convened the 21st meeting of NIMH Alliance for Research Progress (Alliance). In addition to fostering important dialogue between NIMH and stakeholders, these meetings also provide opportunities for Alliance members—leaders from national mental health-related organizations representing patients and their families—to network with colleagues in person and to interact directly with Dr. Insel and senior NIMH staff. The October meeting included presentations and discussions about the NIMH Strategic Plan; the Mood Patient-Powered Research Network—the Patient-Centered Outcomes Research Institute (PCORI) effort most relevant to mental health; Ginger.io and the use of mobile data and behavioral analytics to improve health outcomes; and the Army Study to Assess Risk and Resilience in Servicemembers, a study focusing on mental health resilience and suicide risk among military personnel.
Return to Top
V. Update on Electronic Research Administration (eRA) Activities
- Communication of Policy Changes: The grant application and award processes are complex and dynamic, and communicating critical policy changes is essential. Such communication is formally accomplished through Notices (NOT) published in the NIH Guide to Grants and Contracts . NIH is now providing a new subscription in the Guide. See this tutorial on how to set up a specific search for your needs.
- Finding Information on NIH Web Pages: Here are some suggestions for how to find information within NIH Web pages, user guides, and other resources.
- Each http://grants.nih.gov and http://era.nih.gov Web page has a key word search tool in the upper right corner. Trending search topics are continually analyzed and adjustments are made in an attempt to get users to the wanted results more quickly.
- For most major components of eRA Commons, Online Help is now available. You can access the help systems by going to the eRA Modules, User Guides, and Documentation page and by clicking “Online Help” under each heading. These systems also have a keyword search tool that looks at the contents of the entire help documentation for matches.
- A little less obvious is the Crtl-F keyboard shortcut for Windows (Cmd-F on Mac). By pressing and holding the Crtl key and then pressing the F key, you will bring up a “search this page” feature built into the operating system of the computer. This function works on Microsoft Word documents, PDFs, and Web pages. The function will scan the text for the keyword and highlight it and/or allow you to scroll through each instance.
- New Tutorials Available on the eRA Videos Page: New tutorials include the following: Accessing the Notice of Award (SO & PD/PI) ; Accessing Just-In-Time (SO & PD/PI) ; No-Cost Extension (SO Only) ; Change of Institution (SO & PD/PI) ; and Grants Closeout in eRA Commons . For Peer Reviewers, see these new items: Reviewers: Checking Conflict of Interest in IAR and Reviewers: A Brief Introduction to IAR . Please take advantage of these easy training tools.
- Subaccounting Transition Reminder: It is critical that your institution prepare now for the changes coming in October 2015 regarding the transition of non-competing grant awards from pooled accounts (G accounts) in the Payment Management System (PMS) to subaccounts (P accounts). As NOT-OD-14-103 explained: “Grantees with inadequate systems in place to appropriately manage this transition by October 1, 2015, may be unable to appropriately access PMS accounts and risk losing their ability to draw down funding.”Additional reminders will be sent as the final transition date approaches. Send inquiries to GrantsPolicy@od.nih.gov.
- NIH eSubmission News: For the latest news see NIH eSubmission Items of Interest . The January 7, 2015 edition addresses: the reminder to submit early and check application images; the simplified late policy for application submission; and the ability for single project applications to be submitted through ASSIST.
For more information on all of these updates, please see the NIH eRA News and Events page .
Questions? Contact the eRA help desk . Note that contacting this help desk is the only way to document problems with an electronic grant application submission. Evidence of this contact is the only way to be eligible for any special consideration by the Center for Scientific Review (CSR) Division of Receipt and Referral, should you run into a system problem with Grants.gov or with eRA that is beyond your control.
Return to Top
VI. Research Training and Career Development
Here is the latest news about Research Training and Career Development at the NIMH and the NIH:
- Archived versions of NIMH webinars on the RDoC project and the NIMH Clinical Trials RFAs, held this past autumn, are now available online via the NIMH Training home page. Each webinar provides an overview of the initiative, particularly useful for applicants interested in obtaining a strong foundation of knowledge about these topics. We encourage NIMH mentors and trainees to explore these archived webinars.
- If you’ve ever wanted insights into the NIH grant submission and review processes, you can now explore archived versions of the November 2014 “Meet the Experts in NIH Peer Review” series of webinars. Each webinar focuses on a type of NIH award: research project (R01) grants; fellowship awards; academic research enhancement awards (R15); or, small business grants (SBIR/STTR). You can access the archived video and Powerpoint files, as well as related resources, via the link above.
- As part of our commitment to supporting the research training of physician-scientists, NIMH sponsored a conference for MD/PhD students interested in clinical neuroscience in conjunction with the 2nd Annual Molecular Psychiatry Meeting in November 2014. We would like to congratulate our 12 travel awardees: Abigail Clark (Columbia University), Josh Cohen (University of Alabama at Birmingham), Michelle Corkrum (University of Minnesota Medical School), Robert Corty (University of North Carolina at Chapel Hill), Daniel Fisher (Northwestern University), Eulanca Liu (University of California, San Diego), Joseph Scarpa (Mount Sinai School of Medicine), Nicholas Schwartz (Stony Brook University), Veronica Searles (University of Colorado, Denver), Sara Stockman (University of Maryland School of Medicine), Don Wei (University of California, Irvine) and Aynara Wulsin (University of Cincinnati, College of Medicine). Travel awardees benefited from scientific presentations and career development discussions with the participating faculty and NIMH staff, including Dr. Insel. Travel awardees, in turn, “blitzed” the group with informative and creative three-minute presentations about their own work.
- NIMH recently launched an NIMH Physician-Scientists LinkedIN group, with our 2014 travel awardees as the inaugural members. This closed/invitation-only group encourages networking and active discussions about cutting-edge research, funding opportunities, and professional development relevant to physician-scientists interested in mental health research. Those interested in joining our new LinkedIN group should email Erica Rosemond, PhD for information.
Please contact NIMH Program Staff with questions or comments.
We are interested in feedback from the community; comments or suggestions related to NIMH’s support for research training and career development may be directed to NIMH_Training@mail.nih.gov.
VII. Director’s Blog
The NIMH Director’s Blog provides insights into the latest topics in mental health research:
The following blogs by Former NIMH Director Thomas Insel are no longer available.
- Precision Medicine for Mental Disorders (February 2, 2015): In his latest blog, Dr. Insel discusses precision medicine, which is the new hot topic in research and what it means for mental health.
- The Ignorance Project (January 28, 2015): At the recent World Economic Forum, brain research was a hot topic; Dr. Insel reports on statistics presented at the conference that inspire optimism that progress can be made on difficult problems, including mental disorders.
- Funding Science (January 23, 2015): Relative to other countries, U.S. funding of science has declined in recent years; Dr. Insel talks about the need for research and development related to mental illness.
- What Caused This to Happen? (January 12, 2015): Dr. Insel discusses the idea that chance may have as much to do with the development of mental illness as do genetic and environmental factors.
- Best of 2014 (December 16, 2014): Dr. Insel offers an overview of his top ten mental health stories for 2014.
- Lost in Translation (December 4, 2014): Drug testing in mice has been a poor guide to effectiveness in humans; Dr. Insel talks about the need for research approaches that can more reliably guide medication development.
- Can We Prevent Psychosis? (November 20, 2014): In his blog, Dr. Insel discusses the need for early and accurate prediction of psychosis risk and for effective preventive treatments.
- P-Hacking (November 14, 2014): In his blog, Dr. Insel talks about the reasons for problems with reproducibility in research, among them flawed use of statistical analysis.
- Depression, Daughters and Cellular Aging (October 23, 2014): An early sign of depression risk may provide not only a biomarker for depression but a clue to the relationship between depression and risk for medical illnesses; Dr. Insel blogs.
- Atonement (October 8, 2014): In his blog for Mental Illness Awareness Week, Dr. Insel talks about the complexity of mental disorders and the need for scientists, clinicians, patients, and families to work together in searching for better treatment.
- Ketamine (October 1, 2014): Ongoing research is investigating the long-term efficacy and safety of the anesthetic drug ketamine, which studies have shown can rapidly lift depressive symptoms; Dr. Insel talks about the status of ketamine in his blog.
- From My Data to Mined Data (September 24, 2014): Dr. Insel discusses the importance of data sharing within the scientific community and highlights how NIMH is encouraging this process.
- Childhood and Beyond – Services Research for ASD (September 11, 2014): In his blog, Dr. Insel talks about on new NIMH grants that will support research on services for people of all ages with autism.
- Suicide: a Global Issue (September 4, 2014): Dr. Insel discusses a newly released World Health Organization World Suicide Report, an overview of the impact of and factors involved in suicide globally and strategies for preventing suicide.
- Manipulating Memory (August 28, 2014): Neurotechnologies are making it possible to finely tune brain circuitry to manipulate memory.
Return to Top
VII. NIMH Science Updates
The latest news and updates from NIMH-supported research:
- Disorders Share Risk Gene Pathways for Immune, Epigenetic Regulation (January 29, 2015)
- Webinar Series - Suicide Prevention: An Action Plan to Save Lives (January 23, 2015)
- Brain recalls old memories via new pathways (January 21, 2015)
- Seeking Single Cells’ Secrets (December 30, 2014)
- Despite Risks, Benzodiazepine Use Highest in Older People (December 17, 2014)
- Medications for Patients with First Episode Psychosis May Not Meet Guidelines (December 12, 2014)
- Soldiers at Increased Suicide Risk after Leaving Hosptial (December 12, 2014)
- RDoC Webinar Series Kickoff Event with Dr. Bruce Cuthbert (November 24, 2014)
- Largest Autism Gene Dragnet Fingers 33 Prime Suspects (November 7, 2014)
- NIMH Website Goes Mobile (November 4, 2014)
- NIMH Creates a New Unit to Support Its Research Domain Criteria Initiative (October 30, 2014)
- Rapid Agent Restores Pleasure-seeking Ahead of Other Antidepressant Action (October 17, 2014)
- Groundbreaking Suicide Study (October 10, 2014)
- Increased Health Risks Linked to First-episode Psychosis (October 8, 2014)
- New Report Provides National Clinical Data on the Prevalence of Many Specific Mental Disorders (October 8, 2014)
- NIMH Twitter Chat on Depression and the Development of Novel Medications (October 2, 2014)
- NIH Announces First Wave of Funding for BRAIN Initative Research (October 2, 2014)
- Personalized Screen to ID Suicidal Teens in 14 ERs (September 23, 2014)
- New Grants Fund Cross-lifespan Services research for Autism Spectrum Disorder (September 10, 2014)
- Magnetic Stimulation Boosts Human Memory, Network Connectivity (August 28, 2014)
Publicizing NIMH research is a communal responsibility. Please help us spread the word about the results of NIMH funding by acknowledging our support of your research, for example, in journal articles (citing your NIMH award by number when possible) and other communications. NIMH has two primary methods of getting the word out: press releases and science updates. All releases and updates are posted to the Science News section of the NIMH Web site. These are also distributed to the public through a mailing list .
If you have a manuscript accepted for publication that describes an especially significant finding, please contact your NIMH Program Official to discuss the possibility of a news release or other forms of dissemination.
Return to Top
VIII. Connect with NIMH
Our newest effort to reach our stakeholders is a service that allows you to to subscribe for updates sent directly to your email inbox on the NIMH topics of your choice. In addition to our email newsletters and RSS updates, NIMH offers a vodcast series entitled “Speaking of Science,” and its own YouTube videos on mental health topics. We have also entered the world of Twitter and Facebook , where we highlight Science Updates, Press Releases, and other timely matters.
Inside NIMH is produced by the National Institute of Mental Health. For more information about the Institute, visit our Web site at http://www.nimh.nih.gov. For comments and suggestions about Inside NIMH, please contact the NIMH Webmaster. The material in this newsletter is not copyrighted, and we encourage its use or reprinting.
