2017 Spring Inside NIMH
Welcome
Welcome to the latest edition of Inside NIMH! We publish Inside NIMH in conjunction with each meeting of the National Advisory Mental Health Council, which advises the Secretary of Health and Human Services (HHS), the Director of the National Institutes of Health (NIH), and the Director of NIMH on all policies and activities relating to the conduct and support of mental health research, research training, and other programs of the Institute. In addition, check out our website for regular updates on timely topics at NIMH. I hope you find this information interesting and helpful. Please let us know if you have questions or comments on this edition.
Sincerely,
Joshua A. Gordon, M.D., Ph.D.
Director, National Institute of Mental Health
If you wish to unsubscribe, subscribe, or change your email address, please contact the NIMH Webmaster or visit the Inside NIMH subscription page .
NIMH Director’s Updates
As the spring weather warms, NIMH progress and plans are heating up.
Progress and Plans
- Professional Coalition for Research Progress Convened: NIMH convened the eighth meeting of the Professional Coalition for Research Progress on March 30, 2017. The “Coalition,” which last met in 2011, comprises a group of senior leaders and representatives from national professional organizations with an interest in NIMH research. Attendees learned about current NIMH research directions and strategies, shared the concerns and challenges of members of the organizations they represented, networked with their colleagues, and interacted directly with the NIMH Director and other senior NIMH staff. The meeting included presentations and break-out sessions on the NIMH experimental therapeutics approach to interventions, the Research Domain Criteria (RDoC) initiative, and balancing the NIMH research portfolio.
- National Advisory Mental Health Council (NAMHC) Workgroup Updates:
- A new genomics workgroup will advise the NAMHC on future directions in psychiatric genetics and functional genomics, including how best to address the gap in knowledge between gene discovery and mechanistic models of disease. The workgroup on genomics held their first meeting on March 7, 2017. Meeting participants discussed 1) Issues of design, rigor, and replicability in human genetic studies; 2) Developing appropriate methods for data analysis and modeling; 3) Assuring efficient utilization of data collected with public funds; and 4) Biological interrogation of results from human genetics. The next genomics workgroup meeting will be held on June 19, 2017.
- The workgroup on Opportunities and Challenges of Developing Information Technologies on Behavioral and Social Science Clinical Research is finalizing a report to be presented at the May NAMHC meeting. The report summarizes their discussions of the impact that new mHealth technologies are having on NIMH-related clinical research and includes recommendations for addressing the challenges and opportunities in this rapidly evolving research area.
- The workgroup tasked with evaluating the proposed addition of a motor domain to the RDoC matrix is finalizing a report on their meeting proceedings and alignment of the candidate motor domain constructs with prior elements in the matrix.
- Ethics, Progress, and Future Directions of the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative : On February 14, 2017, the Neuroethics Division of the Multi-Council Working Group (MCWG) of the NIH BRAIN Initiative held its third in-person meeting. Attendees deliberated over how to prepare for future unanticipated ethical concerns, while addressing pragmatic neuroethics questions prompted by current research projects, and discussed the need for neuroethics training for the next generation of neuroscientists. The following day, the full MCWG convened to discuss the current state of the BRAIN Initiative and its future. The directors of the National Institute of Neurological Disorders and Stroke (NINDS) and NIMH, presented on the progress of the BRAIN Initiative. Additional presentations included a report on how the current set of NIH BRAIN Initiative awards align with the roadmap outlined in the BRAIN 2025: A Scientific Vision report and a discussion of evaluation/assessment of the NIH BRAIN Initiative. Participants discussed the importance of evaluating outcomes, supporting dissemination of tools, technology, and data, and bolstering training so scientists can incorporate BRAIN tools and technologies and leverage the data sets in the next stage of the BRAIN Initiative.
- Interagency Autism Coordinating Committee (IACC) Updates: The IACC met on April 26, 2017 and announced the release of two new publications in partnership with the NIMH Office of Autism Research Coordination (OARC). The new publications include the 2016 Summary of Advances in Autism Spectrum Disorder Research and the 2013 IACC Autism Spectrum Disorder Research Portfolio Analysis Report . The IACC also released a new version of the publicly available, online Autism Research Database , which features federally- and privately-funded research from 2008 to 2013. On April 25, 2017, as a special event to recognize Autism Awareness Month in April, OARC hosted a screening of the documentary film “As One: The Autism Project.”
- New Limits on Grant Support Aim to Strengthen Biomedical Workforce and Stewardship: On May 2, 2017, NIH announced a new approach to limit the total NIH grant support provided to an individual principal investigator through NIH-supported research. The approach aims to optimize stewardship of taxpayer dollars by implementing a measure, called the Grant Support Index (GSI). The GSI is a measure of grant support that does not solely focus on money, since different types of science have varying costs. For more information on the implementation of the GSI, see the recent Open Mike blog by Michael Lauer, M.D., Deputy Director for Extramural Research.
- NIH Achieves Milestone to Accelerate Multisite Clinical Studies: The NIH National Center for Advancing Translational Sciences (NCATS) announced that all Clinical and Translational Science Awards Program sites have signed on to the NCATS Streamlined, Multisite, Accelerated Resources for Trials Institution Review Board (SMART IRB) authorization agreement. The SMART IRB authorization agreement — which now includes a total of more than 150 top medical research institutions — will enable all participating study sites to rely on the ethics review of one IRB for each study, making it possible to initiate multisite studies within weeks instead of months. NIH’s policy on single IRB use for multisite studies was designed to improve IRB efficiencies while ensuring the protection of research participants so that research can proceed expeditiously.
- New HHS Secretary’s First Official Visit to NIH: New HHS Secretary Tom Price, M.D., visited the NIH on February 21, 2017. After touring the NIH Clinical Center, Secretary Price tweeted “The @NIH is doing incredible work to improve lives, turning discovery into health. Thank you @NIHDirector and team for the warm welcome.” On March 29, 2017, at a House Appropriations Subcommittee Budget Hearing , Secretary Price stated, “The administration plans on continued investment in high priority mental health issues including suicide prevention, serious mental illness, and children's mental health.”
Budget Overview
-
Fiscal Year (FY) 2017 Budget: On May 5, 2017, President Trump signed the Consolidated Appropriations Act of 2017 (Public Law No. 115-31) providing funds through September 30, 2017. The amount provided to NIMH, $1.602 billion, represents a 3.5 percent increase over the FY 2016 appropriation.
NIMH anticipates awarding more than 600 new and competing research project grants (RPGs) in FY 2017, with an estimated success rate of 23 percent, as can be seen in Figure 1 below.
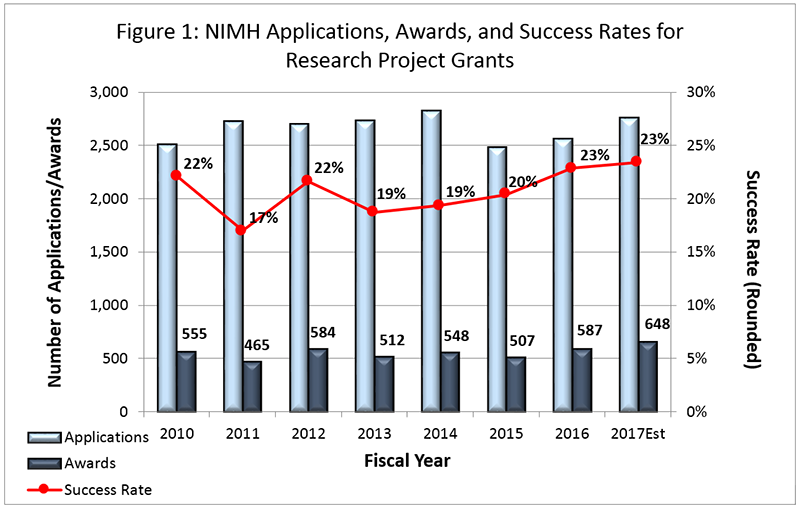
As in past years, NIMH expects to support at least 75 percent of the applications up to the 20th percentile. Moreover, the Institute will give special consideration to applications from Early Stage Investigators . With the exception of specific programmatic adjustments, NIMH will fully fund modular and non-modular grant awards. Future year commitments for modular grant awards are expected to remain consistent with the FY 2017 awarded amount. Non-competing continuation awards from FY 2017 will be made at the committed level, and out-year commitments for continuation awards in FY 2018 and beyond will remain unchanged.
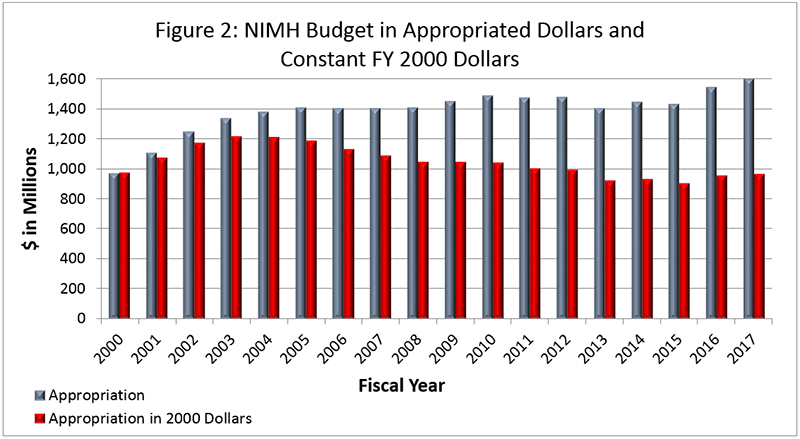
Figure 2 below shows the NIMH budget in appropriated (current) versus constant (FY 2000) dollars. Constant dollars are “inflation adjusted” for variations in the purchasing power of the dollar over time. Dollar amounts are adjusted based on the Biomedical Research and Development Price Index (BRDPI). The annual change in BRDPI indicates how much the NIH budget must change to maintain purchasing power similar to FY 2000.
- Outlook for FY 2018: In May 2017, the President submitted an outline for his FY 2018 budget request to Congress. The request for NIH is $25.9 billion, a decrease of $8.2 billion from the current FY 2017 level. The President's full budget is planned to be released in late May. Congress will likely then begin work on the Labor/HHS/Education FY 2018 appropriations bill, which contains the NIH appropriation.
NIMH Staff News and Awards
- NIMH Staff Awards:
Distinguished Service Award, Society of Neuroimmune Pharmacology- Jeymohan Joseph, Ph.D., Division of AIDS Research
- Jeymohan Joseph, Ph.D., Division of AIDS Research
- We are saddened to announce the passing of one of our former NIMH colleagues:
Martin M. Katz, Ph.D., was a pioneer of psychopharmacology and a key player in NIMH’s history of mental health research. Dr. Katz served as the Chief of the NIMH Clinical Research Branch from 1968 to 1978 and devoted his career to studying the action of antidepressants in clinical populations and the theoretical understanding of their mechanisms of action. Dr. Katz came to the NIH in the late 1950’s to help start the psychopharmacology program, and is credited for helping to develop a behavioral methodology to study the effects of new antidepressant drugs. In 2016, the American College of Neuropsychopharmacology named Dr. Katz the winner of the 2016 Paul Hoch Distinguished Service Award for actively contributing to the college for more than 50 years.
Director’s Highlights: NIMH Scientists and Science
Grantee Awards
NIMH is proud to recognize significant achievement and awards received by our current grantees:
- National Medal of Science
- Mary-Claire King, Ph.D. (University of Washington)
- 2016 Scientific Research Award, National Alliance on Mental Illness (NAMI)
- Steven McCarroll, Ph.D. (Harvard Medical School)
- Michael Carroll, Ph.D. (Boston Children's Hospital)
- Beth Stevens, Ph.D. (Boston Children's Hospital)
- 2017 Fellow of the Biophysical Society Award
- Jonathon Howard, Ph.D. (Yale University)
- 2017 Cognitive Neuroscience Society Young Investigator Award
- Leah H. Somerville, Ph.D. (Harvard University)
- Nicholas Turk-Browne, Ph.D. (Princeton University)
- Newly Elected Members of the American Academy of Arts and Sciences, Neurosciences, Cognitive Sciences, and Behavioral Biology
- Edward S. Boyden, Ph.D. (Massachusetts Institute of Technology)
- Robert T. Knight, M.D. (University of California, Berkeley)
- Helen S. Mayberg, M.D. (Emory University)
- Earl K. Miller, Ph.D. (Massachusetts Institute of Technology)
- William T. Newsome, Ph.D. (Stanford University)
Notable NIMH Grants
The following is a selection of the Institute’s most recently funded projects that exemplify our efforts to accelerate mental health research and to advance the NIMH Strategic Plan for Research:
- Genetic variants (both common and rare mutations) contribute significantly to risk for autism spectrum disorder (ASD). Evan Eichler, Ph.D. (University of Washington), aims to test the significance of rare, gene-disruptive mutations by applying cutting-edge genomic technologies to examine whole-genome sequence data from 35,000 samples from autism families. The proposed study integrates two genetic risk factors (copy number variation and single-nucleotide mutation) for autism to pinpoint likely disease-causing genes. Dr. Eichler’s team plans to select a subset of genes and a subset of families for clinical follow-up to evaluate the characterization of these genes, their expression, and whether the mutations are necessary and sufficient to cause ASD. One of the aims of this study is to generate data and novel analytical pipelines that may improve classification and determination of ASD subtypes based on the genetic variants identified and lead to better diagnosis and treatment development.
- Development of healthy emotion regulation occurs during the pre-school years and may be influenced by maternal input through emotional validation, coaching, and support. Mothers with borderline personality disorder (BPD) frequently have emotion dysregulation, which may have a detrimental effect on their children’s ability to learn how to regulate their emotions. Deficient maternal emotion regulation in women with BPD may be a key environmental factor in the transgenerational transmission of psychopathology in these families. Maureen Zalewski, Ph.D. (University of Oregon), aims to determine if it is possible to restore healthy trajectories of emotion regulation in children by treating mothers with dialectical behavioral therapy, an effective therapy for BPD. This study may identify a modifiable pathway by which maternal BPD places offspring at risk for later mental disorders, and aims quantify how much improvement in children's ability to regulate their emotions can be achieved by treating mothers alone. Knowledge gained may lead to additional prevention interventions focused on a mother to modify a child’s environment.
- The NIMH ServASD II initiative supports research to address the services needs of transition-age youth and adults with ASD. Under this initiative, five new studies will pilot test services that address areas where symptoms of ASD often create barriers to community functioning and integration. Mary Baker, Ph.D. (Rady Children’s Hospital), aims to develop an intervention that fosters development of executive functioning and social cognition to improve vocational and post-secondary educational outcomes. Christina Nicolaidis, M.D., M.P.H. (Portland State University), proposes to refine a computer assisted tool for both primary care providers and adults with ASD to improve their communication and reduce significant healthcare disparities. Tammy Jorgensen-Smith, Ph.D. (South Florida University), aims to develop an intervention that targets vocational rehabilitation specialists to work with both adults with ASD and potential employers in order to achieve and sustain competitive customized employment. Gael Orsmond, Ph.D. (Boston University), plans to test an intervention strategy that engages siblings of adults with ASD in future planning for ongoing support of their family member. These pilot studies aim to inform and refine future full-scale randomized clinical trials of the intervention strategies in these underserved and understudied age groups.
- Depression is one of the most prevalent comorbid mental disorders in the HIV/AIDS population. Depression and HIV infection are marked by systemic inflammation including suppression of innate (natural, non-specific) immunity and over activation of cellular immunity (cellular responses to destroy pathogens, self-destruct, or secrete chemicals to signal other cells). Dwight Evans, M.D. (University of Pennsylvania), and Stephen Douglas, M.D. (Children's Hospital of Philadelphia), are conducting a clinical study to treat depression in people living with HIV using a selective serotonin reuptake inhibitor (SSRI) plus computerized cognitive behavioral therapy (CCBT) compared to CCBT alone. The researchers will assess the effectiveness of SSRI drug therapy to directly increase innate immunity and decrease inflammation in clinically depressed, HIV-infected individuals. Results from this study may lead to use a new use for SSRIs, as adjunctive therapy to modern anti-retroviral therapy, for resolution of depressive symptoms and improved immune function among people living with HIV/AIDS. This clinical study may also contribute to understanding the direct immune benefits of SSRI drug therapy.
For more information on these and other grants selected for funding, please visit the NIH RePORTER website .
Current Funding Opportunities and Announcements
NIH electronically posts the NIH Guide , a listing of all NIH Funding Opportunity Announcements (FOAs) that includes requests for applications (RFAs), program announcements (PAs), and important notices for the scientific community. Below is a selection of recently issued FOAs in which NIMH participates. The Funding page on the NIMH website has links to listings of all NIMH FOAs and other resources.
You can subscribe to the NIMH Funding Opportunities ListServ to receive the latest information about RFAs and other research funding opportunities from NIMH, as well as administrative updates and changes to grant policies and procedures. You can also subscribe to a separate listserv to receive weekly e-mails from the NIH Guide .
NIMH-Administered Requests for Applications
- Limited Competition: National NeuroAIDS Tissue Consortium (NNTC) Clinical Sites
- Release date: April 19, 2017; Application due date: July 26, 2017
- U24 announcement (RFA-MH-18-250 )
- Limited Competition: National NeuroAIDS Tissue Consortium (NNTC) Data Coordinating Center
- Release date: April 19, 2017; Application due date: July 26, 2017
- U24 announcement (RFA-MH-18-251 )
- Role of Myeloid Cells in Persistence and Eradication of HIV-1 Reservoirs from the Brain
- Release date: May 1, 2017; Application due date: September 6, 2017
- R01 announcement (RFA-MH-18-300 )
- R21 announcement (RFA-MH-18-301 )
- BRAIN Initiative: Standards to Define Experiments Related to the BRAIN Initiative
- Release date: September 21, 2016; Application due date: October 11, 2017
- R24 announcement (RFA-MH-17-256 )
- BRAIN Initiative: Development and Validation of Novel Tools to Analyze Cell-Specific and Circuit-Specific Processes in the Brain
- Release date: August 11, 2016; Application due date: October 13, 2017
- R01 announcement (RFA-MH-17-220 )
- BRAIN Initiative: Foundations of Non-Invasive Functional Human Brain Imaging and Recording –Bridging Scales and Modalities
- Release date: August 15, 2016; Application due date: October 13, 2017
- R01 announcement (RFA-MH-17-235 )
- BRAIN Initiative: Non-Invasive Neuromodulation - Mechanisms and Dose/Response Relationships for Targeted CNS Effects
- Release date: August 23, 2016; Application due date: October 13, 2017
- R01 announcement (RFA-MH-17-245 )
- BRAIN Initiative: Non-Invasive Neuromodulation - New Tools and Techniques for Spatiotemporal Precision
- Release date: August 23, 2016; Application due date: October 13, 2017
- R01 announcement (RFA-MH-17-240 )
- BRAIN Initiative Cell Census Network (BICCN) - Specialized Collaboratory on Human and Non-Human Primate Brain Cell Atlases
- Release date: October 19, 2016; Application due date: October 13, 2017
- U01 announcement (RFA-MH-17-210 )
- BRAIN Initiative Cell Census Network (BICCN) - Specialized Collaboratory on Mouse Brain Cell Atlas
- Release date: October 19, 2016; Application due date: October 13, 2017
- U01 announcement (RFA-MH-17-230 )
- BRAIN Initiative: Data Archives for the BRAIN Initiative
- Release date: September 21, 2016; Application due date: October 19, 2017
- R24 announcement (RFA-MH-17-255 )
- BRAIN Initiative: Integration and Analysis of BRAIN Initiative Data
- Release date: September 21, 2016; Application due date: October 26, 2017
- R24 announcement (RFA-MH-17-257 )
- Addressing Suicide Research Gaps: Aggregating and Mining Existing Data Sets for Secondary Analyses
- Release date: May 15, 2017; Application due date: November 2, 2017
- R01 announcement (RFA-MH-18-400 )
- Initiation of a Mental Health Family Navigator Model to Promote Early Access, Engagement and Coordination of Needed Mental Health Services for Children and Adolescents
- Release date: May 2, 2017; Standard due dates apply; Expiration date: January 8, 2018
- R01 announcement (PAR-17-265 )
- R34 announcement (PAR-17-266 )
- Effectiveness Trials for Post-Acute Interventions and Services to Optimize Longer-term Outcomes
- Release date: May 4, 2017; Standard due dates apply; Expiration date: January 24, 2018
- R01 announcement (PAR-17-272)
- R34 announcement (PAR-17-271 )
- Early Stage Testing of Pharmacologic or Device-based Interventions for the Treatment of Mental Disorders
- Release date: December 13, 2016; Application due dates: June 15, 2017 - October 15, 2018
- R61/R33 announcement (RFA-MH-17-600 )
- R33 announcement (RFA-MH-17-602 )
- Development of Psychosocial Therapeutic and Preventive Interventions for Mental Disorders
- Release date: December 13, 2016; Application due dates: June 14, 2017 - October 15, 2018
- R61/R33 announcement (RFA-MH-17-604 )
- R33 announcement (RFA-MH-17-606 )
- Confirmatory Efficacy Clinical Trials of Non-Pharmacological Interventions for Mental Disorders
- Release date: December 13, 2016; Application due dates: June 14, 2017 - October 15, 2018
- R01 announcement (RFA-MH-17-614 )
- Pilot Effectiveness Trials for Treatment, Preventive and Services Interventions
- Release date: December 13, 2016; Application due dates: June 14, 2017 - October 15, 2018
- R34 announcement (RFA-MH-17-612 )
- Clinical Trials to Test the Effectiveness of Treatment, Preventive, and Services Interventions
- Release date: December 13, 2016; Application due dates: June 14, 2017 - October 15, 2018
- Collaborative R01 announcement (RFA-MH-17-610 )
- R01 announcement (RFA-MH-17-608 )
- Reducing the Duration of Untreated Psychosis in the United States
- Release date: May 17, 2016; Standard due dates apply; Expiration date: March 20, 2019
- R01 announcement (PAR-16-265 )
- R34 announcement (PAR-16-264 )
- NIMH Biobehavioral Research Awards for Innovative New Scientists (NIMH BRAINS)
- Release date: February 17, 2017; Application due dates: June 20, 2017 – June 20, 2019
- R01 announcement (RFA-MH-18-200 )
- From Genomic Association to Causation: A Convergent Neuroscience Approach for Integrating Levels of Analysis to Delineate Brain Function in Neuropsychiatry
- Release date: April 11, 2017; Standard due dates apply; Expiration date: September 8, 2020
- R01 announcement (PAR-17-253 )
- Collaborative R01 announcement (PAR-17-252 )
- Innovative Mental Health Services Research Not Involving Clinical Trials
- Release date: April 28, 2017; Standard due dates apply; Expiration date: September 8, 2020
- R01 announcement (PAR-17-264 )
NIMH-Collaborative Requests for Applications
- NHLBI Revision Applications for Regenerative Medicine Innovation Projects (RMIP)
- Release date: April 28, 2017; Application due date: June 26, 2017
- P41 announcement (RFA-HL-17-033 ); P50 announcement (RFA-HL-17-032 ); R01 announcement (RFA-HL-17-029 ); R24 announcement (RFA-HL-17-030 ); R41/42 announcement (RFA-HL-17-024 ); R43/44 announcement (RFA-HL-17-023 ); U01 announcement (RFA-HL-17-034 ); U24 announcement (RFA-HL-17-028 ); U54 announcement (RFA-HL-17-025 ); UC4 announcement (RFA-HL-17-027 ); UM1 announcement (RFA-HL-17-026 ); UM2 announcement (RFA-HL-17-031 )
- BRAIN Initiative: Team-Research BRAIN Circuit Programs - TeamBCP
- Release date: December 2, 2016; Application due date: October 17, 2017
- U19 announcement (RFA-NS-17-018 )
- BRAIN Initiative: Clinical Studies to Advance Next-Generation Invasive Devices for Recording and Modulation in the Human Central Nervous System
- Release date: September 29, 2016; Application due date: October 18, 2017
- UH3 announcement (RFA-NS-17-006 )
- BRAIN Initiative: Next-Generation Invasive Devices for Recording and Modulation in the Human Central Nervous System
- Release date: September 29, 2016; Application due date: October 18, 2017
- U44 announcement (RFA-NS-17-007 )
- UG3/UH3 announcement (RFA-NS-17-005 )
- BRAIN Initiative: New Technologies and Novel Approaches for Large-Scale Recording and Modulation in the Nervous System
- Release date: September 29, 2016; Application due date: October 18, 2017
- U01 announcement (RFA-NS-17-003 )
- BRAIN Initiative: Optimization of Transformative Technologies for Large Scale Recording and Modulation in the Nervous System
- Release date: September 29, 2016; Application due date: October 18, 2017
- U01 announcement (RFA-NS-17-004 )
- BRAIN Initiative: SBIR Direct to Phase II Next-Generation Invasive Devices for Recording and Modulation in the Human Central Nervous System
- Release date: September 29, 2016; Application due date: October 18, 2017
- U44 announcement (RFA-NS-17-008 )
- Silencing of HIV-1 Proviruses
- Release date: May 17, 2017; Application due date: December 6, 2017
- R61/R33 announcement (RFA-AI-17-013 )
- Intensive Longitudinal Analysis of Health Behaviors: Leveraging New Technologies to Understand Health Behaviors
- Release date: March 22, 2017; Application due dates: August 12, 2017 – December 12, 2018.
- U13 announcement (RFA-OD-17-004 )
- U24 announcement (RFA-OD-17-005 ; single application due date: September 25)
Future Research Directions
Concept Clearances for Potential New Research Initiatives
This listing of potential future initiatives is meant to provide the earliest possible alert to the field of our research interests and of potential upcoming announcements to solicit that research. While NIMH plans to proceed with these initiatives, their publication and timing are not certain and depend on sufficient funding. The titles and brief descriptions are consistent with the information available at the time of concept clearance. The resultant FOAs may differ from the concepts in the final wording of their titles or other aspects. To send questions about a specific concept, follow the “Submit Comments” link at the bottom of the description.
- HIV Healthcare Systems Approaches to Improve Viral Suppression (HH-SAIVS)
- Mobile and Connected Health Interventions to Improve Care Continuum and Health Outcomes among Youth with HIV
- Altered Neural Pathways, Receptors and Networks in HIV-induced CNS Dysfunction
For more information, please see recent NAMHC-approved concepts, recent public venue-approved concepts, and past NAMHC meetings, which also contains links to meeting agendas, minutes, and Inside NIMH (Director’s Reports).
NIMH-Sponsored Meetings
- Reducing the Incidence of Suicide in Indigenous Groups: Strengths United through Networks (RISING SUN) – Workshop 3: RISING SUN is an NIMH-led initiative under the 2015-2017 U.S. chairmanship of the Arctic Council and is designed to develop a toolkit of community-based, prioritized outcomes for evaluating suicide prevention interventions among Arctic indigenous communities. The third, and final, RISING SUN workshop was held on March 1-2, 2017, in Iqaluit, Canada. Meeting participants reviewed the international community’s understanding of suicide and learned about activities over the past five years pertaining to evidence gathering and intervention strategies. Findings from the RISING SUN initiative were also presented, including the outcomes from a consensus-building process and in-person meetings. Additionally, meeting participants discussed methodological approaches; the form and function of the RISING SUN toolkit; remaining knowledge gaps; and future opportunities for dissemination, implementation, and research.
- Innovative Measures of Medication Adherence for HIV Treatment and Prevention: On March 13-14, 2017, the NIMH Division of AIDS Research co-sponsored a meeting with the National Institute of Allergy and Infectious Diseases (NIAID) to advance the next-generation of HIV medication adherence measures. The meeting convened eleven research teams who are developing and validating innovative assessments of oral antiretroviral medication adherence under a recent NIMH-NIAID collaborative request for applications (RFA-AI-14-071 ). The meeting highlighted novel drug assays, pill ingestion sensors, and wireless technologies that provide real-time and point-of-care adherence assessments. Meeting discussions centered on opportunities for collaboration and harmonization, and charting the way forward for regulatory approval and clinical use.
- Biometrics and Beyond: Harnessing Computer Vision and Machine Learning to Measure Real World Social Interactions in Psychiatric Populations: On March 27-28, 2017, NIMH, with support from the NIH Office of Behavioral and Social Sciences Research (OBSSR), convened a workshop to address the need for “social biometric” tools to expand the capacity to detect, assess, and monitor both social deficits and the effects of clinical interventions on social behavior. The overall goal of the workshop was to create a community of researchers dedicated to the development of social biometric tools for the collection and analysis of objective, detailed, real-time data in ecological settings. The workshop focused specifically on autism spectrum disorder as a clinical ‘use case.’ Investigators presented current efforts and perceived challenges within and across four areas: emotion/affect, eye gaze/attention, speech/auditory analyses, and body movement.
- Celebrating the Legacy of the NIMH Child Psychiatry Branch, Chief, Judith L. Rapoport, M.D.: On April 17, 2017, the NIMH Division of Intramural Research Programs held a symposium to celebrate decades of neurodevelopmental, neurobiological, and clinical research conducted by the NIMH Child Psychiatry Branch, led by Judith Rapoport, M.D. The symposium brought together leading investigators, mentored by Dr. Rapoport, who continue research into the phenomenology, neurobiology, and treatment of childhood onset psychiatric disorders.
- Transformative Opportunities for Solving the Grand Challenges in Global Mental Health: On May 8-9, 2017, NIMH and Grand Challenges Canada co-convened a global mental health workshop that brought together global mental health researchers, innovators, and other stakeholders. Meeting participants discussed exciting new research findings and strategic opportunities for addressing the six priority areas identified in the Grand Challenges in Global Mental Health initiative. Plenary lectures focused on meeting global mental health priorities through research, policy, and practice; the complexity of getting to root causes in a global context; and the implications of dimensions, diagnoses, and Research Domain Criteria (RDoC) for global mental health research.
- Novel Approaches to Understanding the Mechanisms of the Neuropsychiatric Symptoms in Alzheimer’s and Advancing Therapy Development: In 2005, the FDA issued a black box warning for the use of antipsychotic medications in the elderly. Despite this warning, the use of antipsychotics among older adults has risen, and most prescriptions for these medications are for off label use. The widespread use of these agents points to the need to develop new and better treatments for the behavioral and neuropsychiatric symptoms seen in dementia. On May 8-9, 2017, NIMH hosted a meeting that brought together clinical, behavioral science, and neuroscience researchers to discuss information gaps and the best steps both to improve understanding of the mechanisms of neuropsychiatric symptoms and to further the development of better behavioral and psychopharmacologic treatment approaches.
Electronic Research Administration (eRA) Activities
Electronic Grant Application Submission News
- Reporting Preprints and Other Interim Research Products: For due dates of May 25, 2017 and beyond, interim research products may be cited anywhere other research products are cited in grant applications and progress reports (NOT-OD-17-050 ). The NIH encourages investigators to use interim research products, such as preprints, to speed the dissemination and enhance the rigor of their work. For more information on interim research products, see the FAQs and the recent Open Mike blog by Michael Lauer, M.D., Deputy Director for Extramural Research.
NIH-Wide Grant News
- Interim Guidance on Salary Limitation for Grants and Cooperative Agreements: Current guidance states the amount of direct salary to Executive Level II of the Federal Executive pay is restricted (NOT-OD-17-049 ). The Executive Level II salary was previously set at $185,100, and increased to $187,000 effective January 8, 2017.
- Change to NIH Method of Payment for Awards to Federal Institutions and Individual Fellowships at Federal Sponsoring Institutions: In 2013, NIH announced it would transition payment of awards to federal institutions and individual fellowships at federal sponsoring institutions into Payment Management System subaccounts. However, effective immediately, the NIH Office of Financial Management will continue payment of grants and cooperative agreements to federal departments and agencies through the Interagency Payment and Collection method (NOT-OD-17-052 ). Current federal recipients will receive a revised notice of award pertaining to this change.
For more information on all of these updates, please see the NIH eRA News and Events page .
Questions? Contact the eRA help desk . Note that contacting this help desk is the only way to document problems with an electronic grant application submission. Evidence of this contact is the only way to be eligible for any special consideration by the Center for Scientific Review (CSR) Division of Receipt and Referral, should you run into a system problem with Grants.gov or with eRA that is beyond your control.
Research Training and Career Development
Here is the latest news about Research Training and Career Development at NIMH and NIH:
- Career Development Program in Emergency Care Research: NIMH joined the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Nursing Research (NINR) to support a funding opportunity announcement designed to address the critical need for clinical scientists trained in emergency care research (RFA-HL-16-019 ). NIMH is interested in supporting K12 scholars interested in conducting mentored research in emergency department settings in areas such as: suicide prevention; management of psychiatric emergencies; post-traumatic psychopathology risk detection and intervention; and, mental health services.
Recruitment of the second cohort of K12 scholars will open later in 2017. For more information, interested scholars and mentors can visit the three K12 training sites: Indiana Emergency Care Research (Indiana EMCARE) Training Program (Indiana University School of Medicine), Oregon Emergency Care Research Multidisciplinary Training Program (Oregon Health & Science University), and Vanderbilt Emergency Care Research Training Program (Vanderbilt University).
- Applying for Institutional Training Grants: On March 29, 2017, NIMH held an informational webinar for NIMH Institutional Training Grant (T32) applicants. NIMH training team members presented an overview of the NIMH mission and Strategic Plan for Research. Presenters also reviewed each component of the T32 application, gave rationale for each section, explained required elements, and provided tips for success. The webinar concluded with a question and answer session. Approximately 75 attendees joined the live event.
Please refer to the NIMH webpage for research training and career development between issues of Inside NIMH to locate the latest news and resources for potential applicants and current awardees.
We are interested in feedback from the community; comments or suggestions related to NIMH’s support for research training and career development may be directed to NIMH_Training@mail.nih.gov. You may also contact NIMH Program Staff with questions or comments.
Director’s Messages
The Director’s Messages provide insights into the latest topics in mental health research:
- Towards Interventions Across the Autism Spectrum (April 26, 2017): In the second of two special Director’s Messages about autism spectrum disorder (ASD), NIMH Director Dr. Joshua Gordon talks about NIMH funding of research aimed at developing interventions and services for people across the lifespan with autism spectrum disorder.
- Autism Awareness Month: Genes and Development in Autism Spectrum Disorder (April 4, 2017): In the first of two special Director’s Messages about ASD, NIMH Director Dr. Joshua Gordon discusses what we know so far about what causes ASD, and what NIMH researchers are doing to clarify how these causes lead to symptoms. The message includes findings emerging from studies of genetic factors.
- An Experimental Therapeutic Approach to Psychosocial Interventions (March 20, 2017): Dr. Gordon discusses psychosocial interventions, and how "one possible way forward...emphasizes an experimental therapeutics approach to translating the growing understanding of the factors that cause and sustain mental illnesses into new or improved approaches to prevention and treatment."
NIMH Science News
The latest news and updates from NIMH-supported research:
- NIMH to Host Multimodal Brain Stimulation Speaker Series (May 24, 2017)
- Brain “Relay” Also Key to Holding Thoughts in Mind (May 3, 2017)
- Prescribing Patterns Change Following Direct Marketing Restrictions (May 2, 2017)
- Emergency Departments Could Play Significant Role in Reducing Suicide Attempts (May 1, 2017)
- Human Forebrain Circuits Under Construction – in a Dish (April 26, 2017)
- Estrogen Alters Memory Circuit Function in Women with Gene Variant (April 19, 2017)
- Potential Source of HIV Persistence Confirmed (April 18, 2017)
- Higher Death Rate Among Youth with First Episode Psychosis (April 6, 2017)
- Delayed Walking May Signal Spontaneous Gene Anomalies in Autism (March 24, 2017)
- A Third of Suspect Mutations in ASD Just “Noise” (March 23, 2017)
- Avenevoli Named NIMH Deputy Director (March 17, 2017)
- Sleep May Trim Neural Connections to Restore Learning Ability (February 10, 2017)
Publicizing NIMH research is a communal responsibility. Please help us spread the word about the results of NIMH funding by acknowledging our support of your research, for example, in journal articles (citing your NIMH award by number when possible) and other communications. NIMH has two primary methods of getting the word out: press releases and science updates. All releases and updates are posted to the Science News section of the NIMH Web site. These are also distributed to the public through a mailing list .
Connect with NIMH
Inside NIMH is produced by the National Institute of Mental Health. For more information about the Institute, visit our website at https://www.nimh.nih.gov. For comments and suggestions about Inside NIMH, please contact the NIMH Webmaster. The material in this newsletter is not copyrighted, and we encourage its use or reprinting.
Sign up for the latest mental health news, research advances, upcoming events, publications, clinical trials, meeting summaries, and more. In addition to our email newsletters and RSS updates, please also visit NIMH on Twitter , Facebook , and YouTube , where we highlight Science Updates, Press Releases, and other timely matters.
